Endoderm and Hepatic Progenitor Cells Engraft in the Quiescent Liver Concurrent with Intrinsically Activated Epithelial-to-Mesenchymal Transition
- PMID: 33657866
- PMCID: PMC7940740
- DOI: 10.1177/0963689721993780
Endoderm and Hepatic Progenitor Cells Engraft in the Quiescent Liver Concurrent with Intrinsically Activated Epithelial-to-Mesenchymal Transition
Abstract
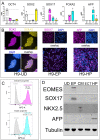
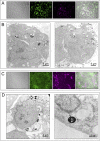
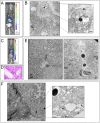
Stem cell transplantation to the liver is a promising therapeutic strategy for a variety of disorders. Hepatocyte transplantation has short-term efficacy but can be problematic due to portal hypertension, inflammation, and sinusoidal thrombosis. We have previously transplanted small mouse endoderm progenitor (EP) cells to successfully reverse a murine model of hemophilia B, and labeling these cells with iron nanoparticles renders them responsive to magnetic fields, which can be used to enhance engraftment. The mechanisms mediating progenitor cell migration from the sinusoidal space to the hepatocyte compartment are unknown. Here we find human EP and hepatic progenitor (HP) cells can be produced from human embryonic stem cells with high efficiency, and they also readily uptake iron nanoparticles. This provides a simple manner through which one can readily identify transplanted cells in vivo using electron microscopy, shortly after delivery. High resolution imaging shows progenitor cell morphologies consistent with epithelial-to-mesenchymal transition (EMT) mediating invasion into the hepatic parenchyma. This occurs in as little as 3 h, which is considerably faster than observed when hepatocytes are transplanted. We confirmed activated EMT in transplanted cells in vitro, as well as in vivo 24 h after transplantation. We conclude that EMT naturally occurs concurrent with EP and HP cell engraftment, which may mediate the rate, safety, and efficacy of early cell engraftment in the undamaged quiescent liver.
Keywords: cell transplantation; endoderm; liver; regenerative medicine; stem cells.
Conflict of interest statement
Figures


References
-
- Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, Ericzon BG. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med. 2012;272(3):201–223. - PubMed
-
- Strom SC, Bruzzone P, Cai H, Ellis E, Lehmann T, Mitamura K, Miki T. Hepatocyte transplantation: clinical experience and potential for future use. Cell Transplant. 2006;15(Suppl 1):S105–S110. - PubMed
-
- Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC, Fox IJ. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003;111(6 Pt 1):1262–1267. - PubMed
-
- Gupta S, Rajvanshi P, Sokhi R, Slehria S, Yam A, Kerr A, Novikoff PM. Entry and integration of transplanted hepatocytes in rat liver plates occur by disruption of hepatic sinusoidal endothelium. Hepatology. 1999;29(2):509–519. - PubMed
-
- Baccarani U, Adani GL, Sanna A, Avellini C, Sainz-Barriga M, Lorenzin D, Montanaro D, Gasparini D, Risaliti A, Donini A, Bresadola F. Portal vein thrombosis after intraportal hepatocytes transplantation in a liver transplant recipient. Transpl Int. 2005;18(6):750–754. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

