Providers' perspectives on the clinical utility of pharmacogenomic testing in pediatric patients
- PMID: 33657875
- PMCID: PMC8050981
- DOI: 10.2217/pgs-2020-0112
Providers' perspectives on the clinical utility of pharmacogenomic testing in pediatric patients
Abstract
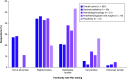
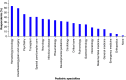
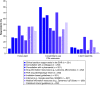
Aim: To assess providers' knowledge, attitudes, perceptions, and experiences related to pharmacogenomic (PGx) testing in pediatric patients. Materials & methods: An electronic survey was sent to multidisciplinary healthcare providers at a pediatric hospital. Results: Of 261 respondents, 71.3% were slightly or not at all familiar with PGx, despite 50.2% reporting prior PGx education or training. Most providers, apart from psychiatry, perceived PGx to be at least moderately useful to inform clinical decisions. However, only 26.4% of providers had recent PGx testing experience. Unfamiliarity with PGx and uncertainty about the clinical value of testing were common perceived challenges. Conclusion: Low PGx familiarity among pediatric providers suggests additional education and electronic resources are needed for PGx examples in which data support testing in children.
Keywords: children; pediatric; perspective; pharmacogenetic; pharmacogenomic; provider; testing.
Conflict of interest statement
This publication is supported in part by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. The contents of this article are the authors’ sole responsibility and do not necessarily represent official NIH views. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No funded writing assistance was utilized in the production of this manuscript.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
