The Kainic Acid Models of Temporal Lobe Epilepsy
- PMID: 33658312
- PMCID: PMC8174050
- DOI: 10.1523/ENEURO.0337-20.2021
The Kainic Acid Models of Temporal Lobe Epilepsy
Abstract
Experimental models of epilepsy are useful to identify potential mechanisms of epileptogenesis, seizure genesis, comorbidities, and treatment efficacy. The kainic acid (KA) model is one of the most commonly used. Several modes of administration of KA exist, each producing different effects in a strain-, species-, gender-, and age-dependent manner. In this review, we discuss the advantages and limitations of the various forms of KA administration (systemic, intrahippocampal, and intranasal), as well as the histologic, electrophysiological, and behavioral outcomes in different strains and species. We attempt a personal perspective and discuss areas where work is needed. The diversity of KA models and their outcomes offers researchers a rich palette of phenotypes, which may be relevant to specific traits found in patients with temporal lobe epilepsy.
Keywords: EEG; hippocampus; kainic acid; mice models of temporal lobe epilepsy.
Copyright © 2021 Rusina et al.
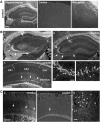
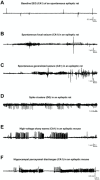
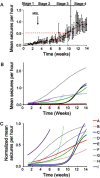
Figures


References
-
- Arabadzisz D, Antal K, Parpan F, Emri Z, Fritschy JM (2005) Epileptogenesis and chronic seizures in a mouse model of temporal lobe epilepsy are associated with distinct EEG patterns and selective neurochemical alterations in the contralateral hippocampus. ExpNeurol 194:76–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
