Functional and Neurochemical Identification of Ghrelin Receptor (GHSR)-Expressing Cells of the Lateral Parabrachial Nucleus in Mice
- PMID: 33658910
- PMCID: PMC7917048
- DOI: 10.3389/fnins.2021.633018
Functional and Neurochemical Identification of Ghrelin Receptor (GHSR)-Expressing Cells of the Lateral Parabrachial Nucleus in Mice
Abstract
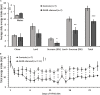
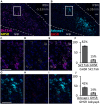
The lateral parabrachial nucleus (lPBN), located in the pons, is a well-recognized anorexigenic center harboring, amongst others, the calcitonin gene-related peptide (CGRP)-expressing neurons that play a key role. The receptor for the orexigenic hormone ghrelin (the growth hormone secretagogue receptor, GHSR) is also abundantly expressed in the lPBN and ghrelin delivery to this site has recently been shown to increase food intake and alter food choice. Here we sought to explore whether GHSR-expressing cells in the lPBN (GHSR lPBN cells) contribute to feeding control, food choice and body weight gain in mice offered an obesogenic diet, involving studies in which GHSR lPBN cells were silenced. We also explored the neurochemical identity of GHSR lPBN cells. To silence GHSR lPBN cells, Ghsr-IRES-Cre male mice were bilaterally injected intra-lPBN with a Cre-dependent viral vector expressing tetanus toxin-light chain. Unlike control wild-type littermates that significantly increased in body weight on the obesogenic diet (i.e., high-fat high-sugar free choice diet comprising chow, lard and 9% sucrose solution), the heterozygous mice with silenced GHSR lPBN cells were resistant to diet-induced weight gain with significantly lower food intake and fat weight. The lean phenotype appeared to result from a decreased food intake compared to controls and caloric efficiency was unaltered. Additionally, silencing the GHSR lPBN cells altered food choice, significantly reducing palatable food consumption. RNAscope and immunohistochemical studies of the lPBN revealed considerable co-expression of GHSR with glutamate and pituitary adenylate cyclase-activating peptide (PACAP), and much less with neurotensin, substance P and CGRP. Thus, the GHSR lPBN cells are important for diet-induced weight gain and adiposity, as well as in the regulation of food intake and food choice. Most GHSR lPBN cells were found to be glutamatergic and the majority (76%) do not belong to the well-characterized anorexigenic CGRP cell population.
Keywords: CGRP; GHSR; PACAP; body weight; food intake and food choice; ghrelin; glutamate; parabrachial nucleus.
Copyright © 2021 Le May, Peris-Sampedro, Stoltenborg, Schéle, Bake, Adan and Dickson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

