Butyrate Shapes Immune Cell Fate and Function in Allergic Asthma
- PMID: 33659009
- PMCID: PMC7917140
- DOI: 10.3389/fimmu.2021.628453
Butyrate Shapes Immune Cell Fate and Function in Allergic Asthma
Abstract
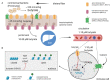
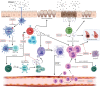
The microbiome plays a fundamental role in how the immune system develops and how inflammatory responses are shaped and regulated. The "gut-lung axis" is a relatively new term that highlights a crucial biological crosstalk between the intestinal microbiome and lung. A growing body of literature suggests that dysbiosis, perturbation of the gut microbiome, is a driving force behind the development, and severity of allergic asthma. Animal models have given researchers new insights into how gut microbe-derived components and metabolites, such as short-chain fatty acids (SCFAs), influence the development of asthma. While the full understanding of how SCFAs influence allergic airway disease remains obscure, a recurring theme of epigenetic regulation of gene expression in several immune cell compartments is emerging. This review will address our current understanding of how SCFAs, and specifically butyrate, orchestrates cell behavior, and epigenetic changes and will provide a detailed overview of the effects of these modifications on immune cells in the context of allergic airway disease.
Keywords: HDAC inhibitor (histone deacetylase inhibitor); SCFA (short chain fatty acids); allergic asthma; butyrate; cell fate and differentiation; epigenetics; inflammation; microbiome.
Copyright © 2021 Yip, Hughes, Li, Cait, Hirst, Mohn and McNagny.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

