Polarization of Macrophages in Insects: Opening Gates for Immuno-Metabolic Research
- PMID: 33659253
- PMCID: PMC7917182
- DOI: 10.3389/fcell.2021.629238
Polarization of Macrophages in Insects: Opening Gates for Immuno-Metabolic Research
Abstract
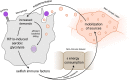
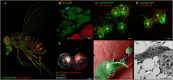
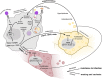
Insulin resistance and cachexia represent severe metabolic syndromes accompanying a variety of human pathological states, from life-threatening cancer and sepsis to chronic inflammatory states, such as obesity and autoimmune disorders. Although the origin of these metabolic syndromes has not been fully comprehended yet, a growing body of evidence indicates their possible interconnection with the acute and chronic activation of an innate immune response. Current progress in insect immuno-metabolic research reveals that the induction of insulin resistance might represent an adaptive mechanism during the acute phase of bacterial infection. In Drosophila, insulin resistance is induced by signaling factors released by bactericidal macrophages as a reflection of their metabolic polarization toward aerobic glycolysis. Such metabolic adaptation enables them to combat the invading pathogens efficiently but also makes them highly nutritionally demanding. Therefore, systemic metabolism has to be adjusted upon macrophage activation to provide them with nutrients and thus support the immune function. That anticipates the involvement of macrophage-derived systemic factors mediating the inter-organ signaling between macrophages and central energy-storing organs. Although it is crucial to coordinate the macrophage cellular metabolism with systemic metabolic changes during the acute phase of bacterial infection, the action of macrophage-derived factors may become maladaptive if chronic or in case of infection by an intracellular pathogen. We hypothesize that insulin resistance evoked by macrophage-derived signaling factors represents an adaptive mechanism for the mobilization of sources and their preferential delivery toward the activated immune system. We consider here the validity of the presented model for mammals and human medicine. The adoption of aerobic glycolysis by bactericidal macrophages as well as the induction of insulin resistance by macrophage-derived factors are conserved between insects and mammals. Chronic insulin resistance is at the base of many human metabolically conditioned diseases such as non-alcoholic steatohepatitis, atherosclerosis, diabetes, and cachexia. Therefore, revealing the original biological relevance of cytokine-induced insulin resistance may help to develop a suitable strategy for treating these frequent diseases.
Keywords: Drosophila; aerobic glycolysis; cachexia; cytokines; immuno-metabolism; insulin resistance; macrophages.
Copyright © 2021 Bajgar, Krejčová and Doležal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Allee J. P. (2011). ImpL2 Represses Insulin Signaling in Response to Hypoxia, Thesis, University of Oregon, Eugene, OR.
-
- Almajwal A., Alam I., Zeb F., Fatima S. (2019). Energy metabolism and allocation in selfish immune system and brain: a beneficial role of insulin resistance in aging. Food Nutr. Sci. 10 64–80. 10.4236/fns.2019.101006 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

