Identification of Socially-activated Neurons
- PMID: 33659404
- PMCID: PMC7842388
- DOI: 10.21769/BioProtoc.3744
Identification of Socially-activated Neurons
Abstract
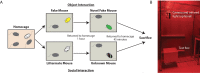
Determining the neuronal circuitry responsible for specific behaviors is a major focus in the field of neurobiology. Activity-dependent immediate early genes (IEGs), transcribed and translated shortly after neurons discharge action potentials, have been used extensively to either identify or gain genetic access to neurons and brain regions involved in such behaviors. By using immunohistochemistry for the protein product of the IEG c-Fos combined with retrograde labeling of specific neuronal populations, precise experimental timing, and identical data acquisition and processing, we present a method to quantitatively identify specific neuronal subpopulations that were active during social encounters. We have previously used this method to show a stronger recruitment of ventral hippocampal neurons that project to the medial prefrontal cortex, compared to those that project to the lateral hypothalamus, following social interactions. After optimization of surgeries for the injection of retrograde tracers, this method will be useful for the identification and mapping of neuronal populations engaged in many different behaviors.
Keywords: Confocal microscopy; Multicolor immunohistochemistry; Neuronal activity; Retrograde tracers; Social behavior; c-Fos.
Copyright © The Authors; exclusive licensee Bio-protocol LLC.
Conflict of interest statement
Competing interestsThe authors declare no competing financial interests.
Figures
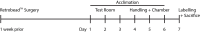

References
-
- Bird C. M. and Burgess N.(2008). The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci 9(3): 182-194. - PubMed
-
- Eun S. Y., Hong Y. H., Kim E. H., Jeon H., Suh Y. H., Lee J. E., Jo C., Jo S. A. and Kim J.(2004). Glutamate receptor-mediated regulation of c-fos expression in cultured microglia. Biochem Biophys Res Commun 325(1): 320-327. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

