Analysis of B Cell Migration by Intravital Microscopy
- PMID: 33659491
- PMCID: PMC7842536
- DOI: 10.21769/BioProtoc.3842
Analysis of B Cell Migration by Intravital Microscopy
Abstract
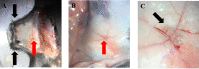
During immune responses, B cells home to lymph nodes (LNs), where they encounter antigens. Homing starts with capture and L-selectin-dependent rolling on the activated endothelium of high endothelial venules (HEV). After recognition of chemokines presented on HEV, activation of B cell integrins occurs mediating firm arrest. Subsequently, B cells crawl to the spot of extravasation to enter the LN. Extravasation can be visualized and quantified in vivo by intravital microscopy (IVM) of the inguinal LN. Here, we describe an established protocol that permits detailed in vivo analysis of B cell recruitment to LN under sterile inflammatory conditions. We describe data acquisition, exportation, quantification, and statistical analysis using specialized software. IVM of LN is a powerful technique that can provide a better understanding of B cell migratory behavior during inflammation in vivo.
Keywords: B cells; Extravasation; Intravital microscopy; Lymph node; Migration; Rolling.
Copyright © 2020 The Authors; exclusive licensee Bio-protocol LLC.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





References
-
- Chayen A. and Parkhouse R. M.(1982). B cell subpopulations in the mouse: analysis with monoclonal antibodies NIM-R2 and NIM-R3. Eur J Immunol 12(9): 725-732. - PubMed
-
- Gavins F. N. and Chatterjee B. E.(2004). Intravital microscopy for the study of mouse microcirculation in anti-inflammatory drug research: focus on the mesentery and cremaster preparations. J Pharmacol Toxicol Methods 49(1): 1-14. - PubMed
-
- Giron-Perez D. A., Vadillo E., Schnoor M. and Santos-Argumedo L.(2020). Myo1e modulates the recruitment of activated B cells to inguinal lymph nodes. J Cell Sci 133(5). - PubMed
LinkOut - more resources
Full Text Sources

