Design of a randomized controlled trial of smoking cessation medications for alcohol reduction among HIV-positive heavy drinkers and daily smokers in St. Petersburg, Russia
- PMID: 33659761
- PMCID: PMC7889999
- DOI: 10.1016/j.conctc.2020.100625
Design of a randomized controlled trial of smoking cessation medications for alcohol reduction among HIV-positive heavy drinkers and daily smokers in St. Petersburg, Russia
Abstract
Background: HIV, heavy drinking, and smoking are all pro-inflammatory and increase risk for coronary heart disease (CHD). Interventions that reduce alcohol use, smoking, or both in HIV-positive people could lower inflammation, CHD and death risk. Varenicline and cytisine are proven therapies for smoking cessation and may also reduce alcohol consumption. The comparative efficacy of varenicline and cytisine to reduce alcohol consumption has not been tested, nor has their comparative effectiveness been reported for smoking.
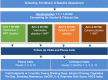
Objective: This paper describes the protocol of the Studying Partial agonists for Ethanol and Tobacco Elimination in Russians with HIV (St PETER HIV), a four-arm parallel-group randomized controlled trial comparing effects of varenicline, cytisine, and nicotine replacement therapy (NRT).
Methods: The study is recruiting four hundred HIV-positive heavy drinking smokers interested in cutting down on alcohol and/or tobacco in St. Petersburg, Russia. Participants are randomly assigned to receive either active varenicline + NRT placebo, varenicline placebo + active NRT, active cytisine + NRT placebo, cytisine placebo + active NRT. All participants receive evidence-based counseling for alcohol and tobacco use, one active medication, and one placebo. Outcomes are: 1) % heavy drinking days in the past month (primary study outcome at three months) and alcohol craving; 2) cigarettes per day (primary smoking outcome at 3 months) and 7-day point prevalence abstinence and; 3) inflammation, CHD risk, and mortality risk.
Conclusion: St PETER HIV addresses the paucity of randomized controlled trial data to guide treatment of alcohol consumption and smoking in HIV-positive heavy drinking smokers.
Keywords: Alcohol use; Cytisine; HIV; Russia; Smoking; Varenicline.
© 2020 Published by Elsevier Inc.
Conflict of interest statement
Dr. Tindle has provided scientific input into the design of a phase III trial for cytisine to be considered for approval by the United States Food and Drug Administration to aid in smoking cessation. In this capacity she has served as an unpaid consultant to Achieve Life Sciences. She is also the principal investigator of an NIH-supported study that has received donated varenicline medication from the manufacturer (Pfizer) for smokers with cancer. The remaining authors report no declarations of interest.
Figures
References
-
- Kuller L., SMART Study Group . Paper Presented at: 15th Conference on Retroviruses and Opportunistic Infections. 2008. Elevated levels of interleukin-6 and D-dimer are associated with an increased risk of death in patients with HIV.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous