Oral Semaglutide Reduces HbA1c and Body Weight in Patients with Type 2 Diabetes Regardless of Background Glucose-Lowering Medication: PIONEER Subgroup Analyses
- PMID: 33660198
- PMCID: PMC7994454
- DOI: 10.1007/s13300-020-00994-9
Oral Semaglutide Reduces HbA1c and Body Weight in Patients with Type 2 Diabetes Regardless of Background Glucose-Lowering Medication: PIONEER Subgroup Analyses
Abstract
Introduction: The efficacy and safety of oral semaglutide, the first oral glucagon-like peptide-1 receptor agonist, were investigated in patients with type 2 diabetes (T2D) in the Peptide InnOvatioN for Early diabEtes tReatment (PIONEER) programme. The current post-hoc exploratory subgroup analyses evaluated outcomes by background medication and insulin regimen subgroups.
Methods: Data from patients in the PIONEER 3-5, 7 and 8 trials receiving once-daily oral semaglutide (14 mg/flexibly dosed) or a comparator (placebo, sitagliptin 100 mg or liraglutide 1.8 mg) were analysed for efficacy (glycated haemoglobin [HbA1c] and body weight changes from baseline to planned end of treatment) and safety outcomes. Patients were grouped according to background medication (metformin, sulphonylurea, thiazolidinedione, sodium-glucose cotransporter-2 inhibitor, insulin, or combinations thereof). Efficacy outcomes were analysed using the trial product estimand (which assumes that patients remained on the trial product without rescue medication use). A separate analysis by background insulin regimen (basal, premixed or basal-bolus) was done for PIONEER 8 using the treatment policy estimand (regardless of trial product discontinuation or rescue medication use). Safety outcomes were analysed descriptively for all patients.
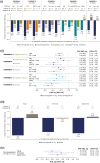
Results: In total, 2836 patients receiving oral semaglutide 14 mg/flexibly dosed or comparators were included. Baseline characteristics were generally similar across background medication subgroups within each trial. Diabetes duration tended to be longer in patients receiving more background medications. Greater HbA1c and body weight reductions were seen across background medication subgroups with oral semaglutide (changes from baseline: - 1.0 to - 1.5% and - 2.2 to - 5.0 kg, respectively) than with comparators (except for similar HbA1c reductions vs liraglutide). There were no statistically significant interactions by treatment and background medication subgroup for change in HbA1c or body weight except for change in HbA1c (background insulin vs insulin plus metformin) in PIONEER 8 (p = 0.0408). Changes in HbA1c and body weight were generally similar across insulin regimen subgroups, without significant treatment interactions by subgroup, and the total daily insulin dose was decreased for patients receiving oral semaglutide. The incidence of adverse events was generally similar in background medication subgroups.
Conclusion: Oral semaglutide was effective at lowering HbA1c and body weight, regardless of background medications, and appears suitable for a broad range of patients with T2D in combination with other glucose-lowering agents.
Trial registration: Clinicaltrials.gov: NCT02607865 (PIONEER 3), NCT02863419 (PIONEER 4), NCT02827708 (PIONEER 5), NCT02849080 (PIONEER 7) and NCT03021187 (PIONEER 8).
Keywords: Diabetes mellitus, type 2; Glucagon-like peptides; Hypoglycaemic agents; Insulin; Metformin; Oral semaglutide; Sodium-glucose cotransporter 2 inhibitors; Sulphonylurea compounds.
Figures

References
-
- Novo Nordisk A/S. Rybelsus®: US prescribing information 2020. https://www.novo-pi.com/rybelsus.pdf. Accessed 30 June 2020.
-
- Novo Nordisk A/S. Rybelsus®: EU summary of product characteristics 2020. https://www.ema.europa.eu/en/documents/product-information/rybelsus-epar.... Accessed 21 Aug 2020.
-
- Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–2701. doi: 10.2337/dci18-0033. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

