Changes in clinical trials of endocrine disorder and metabolism and nutrition disorder drugs in mainland China over 2010-2019
- PMID: 33660404
- PMCID: PMC7931124
- DOI: 10.1002/prp2.729
Changes in clinical trials of endocrine disorder and metabolism and nutrition disorder drugs in mainland China over 2010-2019
Abstract
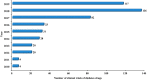
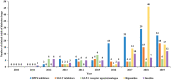
With the improvements in relevant policies, laws, and regulations regarding drug clinical trials in China, the quantity and quality of drug clinical trials have gradually improved, and the development prospects of drug clinical trials for endocrine disorders and metabolism and nutrition disorders are promising. Based on information from the clinical trials from the online drug clinical trial registration platform of the National Medical Products Administration, we aimed to review and evaluate the development of clinical trials of drugs for endocrine disorders and metabolism and nutrition disorders in mainland China from 2010 to 2019, as well as the trends over time. A total of 861 trials were carried out on 254 types of drugs for endocrine disorders and metabolism and nutrition disorders, among which 531 (61.67%) involved endocrine disorders, and 330 (38.33%) addressed metabolism and nutrition disorders. The annual number of clinical trials has been increasing gradually, with a significant increase in 2017. Among them, the proportion of clinical trials with Chinese epidemiological characteristics was relatively large (Wu, Annual Report on Development Health Management and Health Industry in China, 2018). The largest number of trials were for diabetes drugs (55.63%), followed by trials of drugs for hyperlipidemia (19.4%) and those for hyperuricemia (7.9%). It was found that the geographical area of the leading units also showed obvious unevenness according to the analysis of the test unit data. Based on the statistics and evaluation of the data, comprehensive information is provided to support the cooperation of global pharmaceutical R&D companies and research units in China and the development of international multicenter clinical trials in China. This work additionally provides clinical trial units with a self-evaluation of scientific research competitiveness and hospital development strategies. At the same time, it provides a reference with basic data for sponsors and stakeholders in these trials to determine their development strategy goals.
Keywords: China; clinical trial; endocrine disorder; metabolism and nutrition disorder.
© 2021 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
No.
Figures



Similar articles
-
Changes in clinical trials of cancer drugs in mainland China over the decade 2009-18: a systematic review.Lancet Oncol. 2019 Nov;20(11):e619-e626. doi: 10.1016/S1470-2045(19)30491-7. Lancet Oncol. 2019. PMID: 31674320
-
Trend of drug clinical trials in mainland China from 2009 to 2020.Curr Med Res Opin. 2022 Sep;38(9):1499-1507. doi: 10.1080/03007995.2022.2103960. Epub 2022 Jul 29. Curr Med Res Opin. 2022. PMID: 35855662 Review.
-
From past to present: tracing the trends of diabetes drug trials in mainland China.Front Endocrinol (Lausanne). 2025 Jan 10;15:1427148. doi: 10.3389/fendo.2024.1427148. eCollection 2024. Front Endocrinol (Lausanne). 2025. PMID: 39866740 Free PMC article.
-
Evolution of ischemic stroke drug clinical trials in mainland China from 2005 to 2021.CNS Neurosci Ther. 2022 Aug;28(8):1229-1239. doi: 10.1111/cns.13867. Epub 2022 Jun 1. CNS Neurosci Ther. 2022. PMID: 35642775 Free PMC article.
-
Changes in Clinical Trials of Dermatological Drugs in Mainland China Between 2016 and 2022: A Narrative Review.Ther Innov Regul Sci. 2025 May;59(3):450-461. doi: 10.1007/s43441-025-00743-9. Epub 2025 Feb 13. Ther Innov Regul Sci. 2025. PMID: 39948234 Review.
Cited by
-
Characteristics and Trends in Clinical Trials of Cardiovascular Drugs in China from 2009 to 2021.Am J Cardiovasc Drugs. 2023 May;23(3):301-310. doi: 10.1007/s40256-023-00575-8. Epub 2023 Mar 14. Am J Cardiovasc Drugs. 2023. PMID: 36917444
-
Efficacy and safety of Danggui Niantong Decoction in patients with gout: a systematic review and meta-analysis.Front Pharmacol. 2023 Jul 26;14:1168863. doi: 10.3389/fphar.2023.1168863. eCollection 2023. Front Pharmacol. 2023. PMID: 37587984 Free PMC article.
-
The changing landscape of drug clinical trials on cardiometabolic diseases in China, 2009-2021.Diabetol Metab Syndr. 2023 Apr 1;15(1):66. doi: 10.1186/s13098-023-01043-8. Diabetol Metab Syndr. 2023. PMID: 37005689 Free PMC article.
References
-
- Wu LX. Annual Report on Development Health Management and Health Industry in China. Beijing: Zhongguancun Xinzhiyuan Health Management Institute, Central South University Health Management Research Center and Social Sciences Academic Press; 2018.
-
- Liu M, Liu S‐W, Wang L‐J, et al. Burden of diabetes, hyperglycaemia in China from to 2016: findings from the 1990 to 2016, global burden of disease study. Diabetes Metab. 2019;45(3):286‐293. - PubMed
-
- Zhao S, Lv C, Gong JF, et al. Challenges in anticancer drug R&D in China. Lancet Oncol. 2019;20(2):183‐186. - PubMed
-
- Wang M. Clinical trials and drug approvals continue to accelerate in China. Lancet Oncol. 2017;18(7):855. - PubMed
-
- Zhou Q, Chen X‐Y, Yang Z‐M, et al. The changing landscape of clinical trial and approval processes in China. Nat Rev Clin Oncol. 2017;14(9):577‐583. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

