Bingeing on High-Fat Food Enhances Evoked Dopamine Release and Reduces Dopamine Uptake in the Nucleus Accumbens
- PMID: 33660412
- PMCID: PMC8048651
- DOI: 10.1002/oby.23122
Bingeing on High-Fat Food Enhances Evoked Dopamine Release and Reduces Dopamine Uptake in the Nucleus Accumbens
Abstract
Objective: Binge-eating disorder (BED) disrupts dopamine neuron function, in part by altering dopamine transporter (DAT) activity. This study characterized the effects of high-fat bingeing on presynaptic dopamine terminals and tested the hypothesis that acute low-dose amphetamine would restore DAT function.
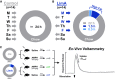
Methods: C57BL/6 mice were given limited access (LimA) to a high-fat diet (2 h/d, 3 d/wk) or standard chow (control). After 6 weeks, ex vivo fast-scan cyclic voltammetry was used to characterize dopamine-terminal adaptations in the nucleus accumbens. Prior to undergoing fast-scan cyclic voltammetry, some mice from each group were given amphetamine (0.5 mg/kg intraperitoneally).
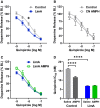
Results: Escalation of high fat intake, termed bingeing, occurred in the LimA group and coincided with increased phasic dopamine release, reduced dopamine uptake rates, and increased dopamine receptor 2 (D2 ) autoreceptor function. Acute amphetamine selectively reversed dopamine uptake changes in the LimA group and restored the potency of amphetamine to inhibit uptake.
Conclusions: High-fat bingeing enhanced dopaminergic signaling in the nucleus accumbens by promoting phasic dopamine release and reducing clearance. This study's data show that amphetamine was efficacious in restoring impaired DAT function caused by high-fat bingeing but did not reduce dopamine release to normal. These presynaptic changes should be considered if amphetamine-like dopamine releasers are used as treatments for BED.
© 2021 The Authors. Obesity published by Wiley Periodicals LLC on behalf of The Obesity Society (TOS).
Conflict of interest statement
The authors declared no conflict of interest.
Figures




Similar articles
-
High fat diet augments amphetamine sensitization in mice: Role of feeding pattern, obesity, and dopamine terminal changes.Neuropharmacology. 2016 Oct;109:170-182. doi: 10.1016/j.neuropharm.2016.06.006. Epub 2016 Jun 4. Neuropharmacology. 2016. PMID: 27267686 Free PMC article.
-
Reduced phasic dopamine release and slowed dopamine uptake occur in the nucleus accumbens after a diet high in saturated but not unsaturated fat.Nutr Neurosci. 2022 Jan;25(1):33-45. doi: 10.1080/1028415X.2019.1707421. Epub 2020 Jan 9. Nutr Neurosci. 2022. PMID: 31914869 Free PMC article.
-
Diazepam Inhibits Electrically Evoked and Tonic Dopamine Release in the Nucleus Accumbens and Reverses the Effect of Amphetamine.ACS Chem Neurosci. 2017 Feb 15;8(2):300-309. doi: 10.1021/acschemneuro.6b00358. Epub 2017 Jan 26. ACS Chem Neurosci. 2017. PMID: 28038309
-
Amphetamine potency varies with dopamine uptake rate across striatal subregions.J Neurochem. 2014 Nov;131(3):348-55. doi: 10.1111/jnc.12808. Epub 2014 Jul 30. J Neurochem. 2014. PMID: 24988947 Free PMC article.
-
Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter.J Neurosci. 1998 Mar 15;18(6):1979-86. doi: 10.1523/JNEUROSCI.18-06-01979.1998. J Neurosci. 1998. PMID: 9482784 Free PMC article. Review.
Cited by
-
The Rise and Fall of Dopamine: A Two-Stage Model of the Development and Entrenchment of Anorexia Nervosa.Front Psychiatry. 2022 Jan 11;12:799548. doi: 10.3389/fpsyt.2021.799548. eCollection 2021. Front Psychiatry. 2022. PMID: 35087433 Free PMC article.
-
Chronic Exposure to High Fat Diet Affects the Synaptic Transmission That Regulates the Dopamine Release in the Nucleus Accumbens of Adolescent Male Rats.Int J Mol Sci. 2023 Feb 28;24(5):4703. doi: 10.3390/ijms24054703. Int J Mol Sci. 2023. PMID: 36902133 Free PMC article.
-
Dopaminergic Perturbation in the Aetiology of Neurodevelopmental Disorders.Mol Neurobiol. 2025 Feb;62(2):2420-2434. doi: 10.1007/s12035-024-04418-8. Epub 2024 Aug 7. Mol Neurobiol. 2025. PMID: 39110391 Review.
-
The Role of Mu Opioid Receptors in High Fat Diet-Induced Reward and Potentiation of the Rewarding Effect of Oxycodone.Life (Basel). 2023 Feb 23;13(3):619. doi: 10.3390/life13030619. Life (Basel). 2023. PMID: 36983775 Free PMC article.
-
Healthful vs. Unhealthful Plant-Based Restaurant Meals.Nutrients. 2025 Feb 20;17(5):742. doi: 10.3390/nu17050742. Nutrients. 2025. PMID: 40077611 Free PMC article.
References
-
- Corwin RL, Babbs RK. Rodent models of binge eating: are they models of addiction? ILAR J 2012;53:23‐34. - PubMed
-
- Naef L, Pitman KA, Borgland SL. Mesolimbic dopamine and its neuromodulators in obesity and binge eating. CNS Spectr 2015;20:574‐583. - PubMed
-
- Davis C, Levitan RD, Kaplan AS, et al. Dopamine transporter gene (DAT1) associated with appetite suppression to methylphenidate in a case‐control study of binge eating disorder. Neuropsychopharmacology 2007;32:2199‐2206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01DA048490/DA/NIDA NIH HHS/United States
- U01 AA014091/AA/NIAAA NIH HHS/United States
- R01 AA023999/AA/NIAAA NIH HHS/United States
- P50AA026117/AA/NIAAA NIH HHS/United States
- R15DK119897/DK/NIDDK NIH HHS/United States
- U01AA014091/AA/NIAAA NIH HHS/United States
- R01 DA048490/DA/NIDA NIH HHS/United States
- P50DA006634/DA/NIDA NIH HHS/United States
- P50 AA026117/AA/NIAAA NIH HHS/United States
- T32 AA007565/AA/NIAAA NIH HHS/United States
- R15 DK119897/DK/NIDDK NIH HHS/United States
- P50 DA006634/DA/NIDA NIH HHS/United States
- T32 DA041349/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

