Intraindividual Comparison of Hepatocellular Carcinoma Washout between MRIs with Hepatobiliary and Extracellular Contrast Agents
- PMID: 33660458
- PMCID: PMC8076831
- DOI: 10.3348/kjr.2020.1143
Intraindividual Comparison of Hepatocellular Carcinoma Washout between MRIs with Hepatobiliary and Extracellular Contrast Agents
Abstract
Objective: To intraindividually compare hepatocellular carcinoma (HCC) washout between MRIs using hepatobiliary agent (HBA) and extracellular agent (ECA).
Materials and methods: This study included 114 prospectively enrolled patients with chronic liver disease (mean age, 55 ± 9 years; 94 men) who underwent both HBA-MRI and ECA-MRI before surgical resection for HCC between November 2016 and May 2019. For 114 HCCs, the lesion-to-liver visual signal intensity ratio (SIR) using a 5-point scale (-2 to +2) was evaluated in each phase. Washout was defined as negative visual SIR with temporal reduction of visual SIR from the arterial phase. Illusional washout (IW) was defined as a visual SIR of 0 with an enhancing capsule. The frequency of washout and MRI sensitivity for HCC using LR-5 or its modifications were compared between HBA-MRI and ECA-MRI. Subgroup analysis was performed according to lesion size (< 20 mm or ≥ 20 mm).
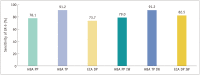
Results: The frequency of portal venous phase (PP) washout with HBA-MRI was comparable to that of delayed phase (DP) washout with ECA-MRI (77.2% [88/114] vs. 68.4% [78/114]; p = 0.134). The frequencies were also comparable when IW was allowed (79.8% [91/114] for HBA-MRI vs. 81.6% [93/114] for ECA-MRI; p = 0.845). The sensitivities for HCC of LR-5 (using PP or DP washout) were comparable between HBA-MRI and ECA-MRI (78.1% [89/114] vs. 73.7% [84/114]; p = 0.458). In HCCs < 20 mm, the sensitivity of LR-5 was higher on HBA-MRI than on ECA-MRI (70.8% [34/48] vs. 50.0% [24/48]; p = 0.034). The sensitivity was similar to each other if IW was added to LR-5 (72.9% [35/48] for HBA-MRI vs. 70.8% [34/48] for ECA-MRI; p > 0.999).
Conclusion: Extracellular phase washout for HCC diagnosis was comparable between MRIs with both contrast agents, except for tumors < 20 mm. Adding IW could improve the sensitivity for HCC on ECA-MRI in tumors < 20 mm.
Keywords: Extracellular contrast; Gadoxetic acid; Hepatocellular carcinoma; Magnetic resonance imaging; Washout.
Copyright © 2021 The Korean Society of Radiology.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures



Similar articles
-
Prospective Intraindividual Comparison of Magnetic Resonance Imaging With Gadoxetic Acid and Extracellular Contrast for Diagnosis of Hepatocellular Carcinomas Using the Liver Imaging Reporting and Data System.Hepatology. 2018 Dec;68(6):2254-2266. doi: 10.1002/hep.30122. Epub 2018 Nov 12. Hepatology. 2018. PMID: 30070365
-
Comparison of extracellular and hepatobiliary MR contrast agents for the diagnosis of small HCCs.J Hepatol. 2020 May;72(5):937-945. doi: 10.1016/j.jhep.2019.12.011. Epub 2019 Dec 21. J Hepatol. 2020. PMID: 31870951 Clinical Trial.
-
A modified LI-RADS: targetoid tumors with enhancing capsule can be diagnosed as HCC instead of LR-M lesions.Eur Radiol. 2022 Feb;32(2):912-922. doi: 10.1007/s00330-021-08124-0. Epub 2021 Aug 4. Eur Radiol. 2022. PMID: 34345947
-
Diagnostic performance of MRI for HCC according to contrast agent type: a systematic review and meta-analysis.Hepatol Int. 2020 Dec;14(6):1009-1022. doi: 10.1007/s12072-020-10100-7. Epub 2020 Nov 4. Hepatol Int. 2020. PMID: 33146841
-
Diagnostic performance of MRI using extracellular contrast agents versus gadoxetic acid for hepatocellular carcinoma: A systematic review and meta-analysis.Liver Int. 2021 May;41(5):1117-1128. doi: 10.1111/liv.14850. Epub 2021 Mar 8. Liver Int. 2021. PMID: 33647177
Cited by
-
Comparison of Gadoxetic Acid-Enhanced Liver Magnetic Resonance Imaging and Contrast-Enhanced Computed Tomography for the Noninvasive Diagnosis of Hepatocellular Carcinoma.Liver Cancer. 2025 Apr 22:1-13. doi: 10.1159/000545965. Online ahead of print. Liver Cancer. 2025. PMID: 40487794 Free PMC article.
-
Magnetic resonance imaging for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease.Cochrane Database Syst Rev. 2022 May 6;5(5):CD014798. doi: 10.1002/14651858.CD014798.pub2. Cochrane Database Syst Rev. 2022. PMID: 35521901 Free PMC article.
-
Value of enhancing capsule for diagnosing hepatocellular carcinoma on MRI: implications for simplifying LI-RADS.Eur Radiol. 2025 Aug 22. doi: 10.1007/s00330-025-11938-x. Online ahead of print. Eur Radiol. 2025. PMID: 40847079
-
Imaging diagnosis of hepatocellular carcinoma: Future directions with special emphasis on hepatobiliary magnetic resonance imaging and contrast-enhanced ultrasound.Clin Mol Hepatol. 2022 Jul;28(3):362-379. doi: 10.3350/cmh.2021.0361. Epub 2021 Dec 27. Clin Mol Hepatol. 2022. PMID: 34955003 Free PMC article. Review.
References
-
- Kim YY, Kim MJ, Kim EH, Roh YH, An C. Hepatocellular carcinoma versus other hepatic malignancy in cirrhosis: performance of LI-RADS version 2018. Radiology. 2019;291:72–80. - PubMed
-
- Sofue K, Sirlin CB, Allen BC, Nelson RC, Berg CL, Bashir MR. How reader perception of capsule affects interpretation of washout in hypervascular liver nodules in patients at risk for hepatocellular carcinoma. J Magn Reson Imaging. 2016;43:1337–1345. - PubMed
-
- Stocker D, Becker AS, Barth BK, Skawran S, Kaniewska M, Fischer MA, et al. Does quantitative assessment of arterial phase hyperenhancement and washout improve LI-RADS v2018-based classification of liver lesions? Eur Radiol. 2020;30:2922–2933. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

