Unraveling the interaction between doxorubicin and DNA origami nanostructures for customizable chemotherapeutic drug release
- PMID: 33660776
- PMCID: PMC8034656
- DOI: 10.1093/nar/gkab097
Unraveling the interaction between doxorubicin and DNA origami nanostructures for customizable chemotherapeutic drug release
Abstract
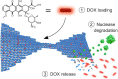
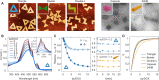
Doxorubicin (DOX) is a common drug in cancer chemotherapy, and its high DNA-binding affinity can be harnessed in preparing DOX-loaded DNA nanostructures for targeted delivery and therapeutics. Although DOX has been widely studied, the existing literature of DOX-loaded DNA-carriers remains limited and incoherent. Here, based on an in-depth spectroscopic analysis, we characterize and optimize the DOX loading into different 2D and 3D scaffolded DNA origami nanostructures (DONs). In our experimental conditions, all DONs show similar DOX binding capacities (one DOX molecule per two to three base pairs), and the binding equilibrium is reached within seconds, remarkably faster than previously acknowledged. To characterize drug release profiles, DON degradation and DOX release from the complexes upon DNase I digestion was studied. For the employed DONs, the relative doses (DOX molecules released per unit time) may vary by two orders of magnitude depending on the DON superstructure. In addition, we identify DOX aggregation mechanisms and spectral changes linked to pH, magnesium, and DOX concentration. These features have been largely ignored in experimenting with DNA nanostructures, but are probably the major sources of the incoherence of the experimental results so far. Therefore, we believe this work can act as a guide to tailoring the release profiles and developing better drug delivery systems based on DNA-carriers.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Seeman N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982; 99:237–247. - PubMed
-
- Jones M.R., Seeman N.C., Mirkin C.A.. Programmable materials and the nature of the DNA bond. Science. 2015; 347:1260901. - PubMed
-
- Hong F., Zhang F., Liu Y., Yan H.. DNA origami: scaffolds for creating higher order structures. Chem. Rev. 2017; 117:12584–12640. - PubMed
-
- Nummelin S., Kommeri J., Kostiainen M.A., Linko V.. Evolution of structural DNA nanotechnology. Adv. Mater. 2018; 30:1703721. - PubMed
-
- Heuer-Jungemann A., Liedl T.. From DNA tiles to functional DNA materials. Trends Chem. 2019; 1:799–814.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

