Complete minicircle genome of Leptomonas pyrrhocoris reveals sources of its non-canonical mitochondrial RNA editing events
- PMID: 33660779
- PMCID: PMC8034629
- DOI: 10.1093/nar/gkab114
Complete minicircle genome of Leptomonas pyrrhocoris reveals sources of its non-canonical mitochondrial RNA editing events
Abstract
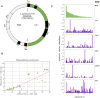
Uridine insertion/deletion (U-indel) editing of mitochondrial mRNA, unique to the protistan class Kinetoplastea, generates canonical as well as potentially non-productive editing events. While the molecular machinery and the role of the guide (g) RNAs that provide required information for U-indel editing are well understood, little is known about the forces underlying its apparently error-prone nature. Analysis of a gRNA:mRNA pair allows the dissection of editing events in a given position of a given mitochondrial transcript. A complete gRNA dataset, paired with a fully characterized mRNA population that includes non-canonically edited transcripts, would allow such an analysis to be performed globally across the mitochondrial transcriptome. To achieve this, we have assembled 67 minicircles of the insect parasite Leptomonas pyrrhocoris, with each minicircle typically encoding one gRNA located in one of two similar-sized units of different origin. From this relatively narrow set of annotated gRNAs, we have dissected all identified mitochondrial editing events in L. pyrrhocoris, the strains of which dramatically differ in the abundance of individual minicircle classes. Our results support a model in which a multitude of editing events are driven by a limited set of gRNAs, with individual gRNAs possessing an inherent ability to guide canonical and non-canonical editing.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Sanford J.R., Caceres J.F.. Pre-mRNA splicing: life at the centre of the central dogma. J. Cell Sci. 2004; 117:6261–6263. - PubMed
-
- Shi Y. Mechanistic insights into precursor messenger RNA splicing by the spliceosome. Nat. Rev. Mol. Cell Biol. 2017; 18:655–670. - PubMed
-
- Lukeš J., Kaur B., Speijer D.. RNA editing in mitochondria and plastids: weird and widespread. Trends Genet. 2021; 37:99–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

