The role of EGFR-TKIs as adjuvant therapy in EGFR mutation-positive early-stage NSCLC: A meta-analysis
- PMID: 33660941
- PMCID: PMC8017245
- DOI: 10.1111/1759-7714.13874
The role of EGFR-TKIs as adjuvant therapy in EGFR mutation-positive early-stage NSCLC: A meta-analysis
Abstract
Background: The role of adjuvant epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) is not clear in early-stage nonsmall-cell lung cancer (NSCLC) patients. This meta-analysis aims to compare the efficacy and safety of EGFR-TKIs as adjuvant therapy with chemotherapy or placebo in NSCLC patients harboring EGFR mutations.
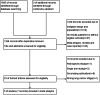
Patients and methods: Pubmed, Embase, and Cochrane databases were searched for randomized controlled trials. The hazard ratio (HR) of disease-free survival (DFS) and overall survival (OS) as well as the risk ratio (RR) of severe adverse events were merged.
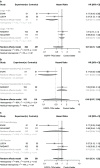
Results: Seven articles from five studies from 1843 records, a total of 1227 patients, were included in the analysis. The HR for DFS was 0.38 (95% confidence interval [CI] 0.22-0.63), in favor of EGFR-TKIs. However, no significant benefit of OS was seen (HR = 0.61, 95% CI 0.31-1.22). Treatment benefit was more pronounced in patients with advanced disease stage and longer duration of medication, EGFR exon 19 deletion mutation, and treatment with third-generation EGFR-TKIs. Adjuvant targeted therapy may cause few adverse events compared with chemotherapy (RR = 0.28, 95% CI 0.09-0.94). The possibility of severe adverse events for the first-generation drugs was significantly lower than for third-generation drugs.
Conclusion: In EGFR mutation-positive patients with stage IB-IIIA NSCLC, compared with adjuvant chemotherapy or placebo, adjuvant EGFR-TKIs should effectively improve the patient's DFS, but not effectively improve OS. Disease stage, treatment duration, mutation types, and therapeutic drugs could affect the degree of benefit. Adjuvant EGFR-TKIs had more favorable tolerability than chemotherapy, especially with the usage of first-generation drugs.
Keywords: adjuvant treatment; epidermal growth factor receptor tyrosine kinase inhibitors; nonsmall-cell lung cancer; survival; targeted therapy.
© 2021 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures




Similar articles
-
EGFR inhibitors as adjuvant therapy for resected non-small cell lung cancer harboring EGFR mutations.Lung Cancer. 2019 Oct;136:6-14. doi: 10.1016/j.lungcan.2019.08.001. Epub 2019 Aug 2. Lung Cancer. 2019. PMID: 31421260
-
Adjuvant Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors (TKIs) in Resected Non-Small Cell Lung Cancer (NSCLC): A Systematic Review and Meta-analysis.Am J Clin Oncol. 2019 May;42(5):440-445. doi: 10.1097/COC.0000000000000533. Am J Clin Oncol. 2019. PMID: 30913091
-
Efficacy and safety of adjuvant EGFR-TKIs for resected non-small cell lung cancer: a systematic review and meta-analysis based on randomized control trials.BMC Cancer. 2022 Mar 26;22(1):328. doi: 10.1186/s12885-022-09444-0. BMC Cancer. 2022. PMID: 35346117 Free PMC article.
-
A meta-analysis of adjuvant EGFR-TKIs for patients with resected non-small cell lung cancer.Lung Cancer. 2019 Nov;137:7-13. doi: 10.1016/j.lungcan.2019.08.002. Epub 2019 Aug 5. Lung Cancer. 2019. PMID: 31520922
-
Rational application of EGFR-TKI adjuvant therapy in patients with completely resected stage IB-IIIA EGFR-mutant NSCLC: a systematic review and meta-analysis of 11 randomized controlled trials.BMC Cancer. 2023 Aug 1;23(1):719. doi: 10.1186/s12885-023-11194-6. BMC Cancer. 2023. PMID: 37528390 Free PMC article.
Cited by
-
EGFR-Mutant Non-Small-Cell Lung Cancer at Surgical Stages: What Is the Place for Tyrosine Kinase Inhibitors?Cancers (Basel). 2022 Apr 30;14(9):2257. doi: 10.3390/cancers14092257. Cancers (Basel). 2022. PMID: 35565386 Free PMC article. Review.
-
ADAURA: The Splash of Osimertinib in Adjuvant EGFR-Mutant Non-small Cell Lung Cancer.Oncol Ther. 2022 Jun;10(1):13-22. doi: 10.1007/s40487-022-00190-8. Epub 2022 Mar 16. Oncol Ther. 2022. PMID: 35294773 Free PMC article.
-
EGFR Status Assessment for Better Care of Early Stage Non-Small Cell Lung Carcinoma: What Is Changing in the Daily Practice of Pathologists?Cells. 2021 Aug 21;10(8):2157. doi: 10.3390/cells10082157. Cells. 2021. PMID: 34440926 Free PMC article. Review.
-
A network meta-analysis of efficacy and safety of adjuvant targeted therapy or immunotherapy in non-small cell lung cancer.Sci Rep. 2025 Jul 1;15(1):21696. doi: 10.1038/s41598-025-05823-2. Sci Rep. 2025. PMID: 40596078 Free PMC article.
-
Prediction of EGFR mutation status in lung adenocarcinoma based on 18F-FDG PET/CT radiomic features.Am J Nucl Med Mol Imaging. 2023 Oct 20;13(5):230-244. eCollection 2023. Am J Nucl Med Mol Imaging. 2023. PMID: 38023818 Free PMC article.
References
-
- Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and National Cancer Incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5:1749–68. - PMC - PubMed
-
- Le Chevalier T. Adjuvant chemotherapy for resectable non‐small‐cell lung cancer: where is it going. Ann Oncol. 2010;21(suppl 7):vii196–8. - PubMed
-
- Cagle PT, Allen TC, Olsen RJ. Lung cancer biomarkers: present status and future developments. Arch Pathol Lab Med. 2013;137:1191–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

