Cardiolipin targets a dynamin-related protein to the nuclear membrane
- PMID: 33661098
- PMCID: PMC7946437
- DOI: 10.7554/eLife.64416
Cardiolipin targets a dynamin-related protein to the nuclear membrane
Abstract
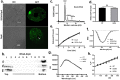
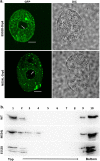
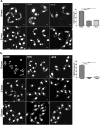
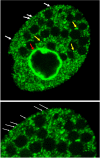
Dynamins are targeted to specific cellular membranes that they remodel via membrane fusion or fission. The molecular basis of conferring specificity to dynamins for their target membrane selection is not known. Here, we report a mechanism of nuclear membrane recruitment of Drp6, a dynamin member in Tetrahymena thermophila. Recruitment of Drp6 depends on a domain that binds to cardiolipin (CL)-rich bilayers. Consistent with this, nuclear localization of Drp6 was inhibited either by depleting cellular CL or by substituting a single amino acid residue that abolished Drp6 interactions with CL. Inhibition of CL synthesis, or perturbation in Drp6 recruitment to nuclear membrane, caused defects in the formation of new macronuclei post-conjugation. Taken together, our results elucidate a molecular basis of target membrane selection by a nuclear dynamin and establish the importance of a defined membrane-binding domain and its target lipid in facilitating nuclear expansion.
Keywords: cell biology; dynamin; lipid protein interaction; membrane remodelling; nuclear remodelling; target membrane selection; tetrahymena.
© 2021, Kar et al.
Conflict of interest statement
UK, HD, AR No competing interests declared
Figures


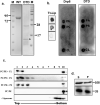
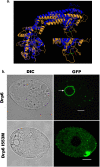

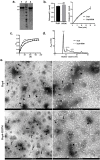
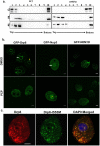

References
-
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Journal of Biochemistry and Physiology. 1959;37:911–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

