Cardio Phenotypic Potential of Mesenchymal Stem Cells
- PMID: 33661576
- PMCID: PMC11494489
- DOI: 10.1002/cpz1.62
Cardio Phenotypic Potential of Mesenchymal Stem Cells
Erratum in
-
Group Correction Statement (Human or Animal Subject Note).Curr Protoc. 2022 Aug;2(8):e554. doi: 10.1002/cpz1.554. Curr Protoc. 2022. PMID: 36005901 No abstract available.
-
Group Correction Statement (Data Availability Statements).Curr Protoc. 2022 Aug;2(8):e552. doi: 10.1002/cpz1.552. Curr Protoc. 2022. PMID: 36005902 Free PMC article. No abstract available.
-
Group Correction Statement (Conflict of Interest Statements).Curr Protoc. 2022 Aug;2(8):e551. doi: 10.1002/cpz1.551. Curr Protoc. 2022. PMID: 36005903 Free PMC article. No abstract available.
Abstract
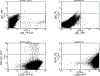
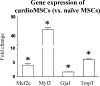
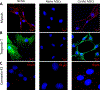
Cell therapy is being investigated as a powerful intervention to ameliorate the consequences of coronary artery disease. Among the different stem cell options, mesenchymal stem cells (MSCs) are particularly attractive due to their high availability, as well as immune-privileged status. However, it is still unclear whether mesenchymal stem cells can acquire cardiomyogenic characteristics after they are transplanted to the myocardium. In this article, we outline protocols that illustrate the plasticity of MSCs and their ability to acquire cardiogenic characteristics when they are in an ischemic-like environment, as typically encountered after transplantation into the ischemic heart. © 2021 Wiley Periodicals LLC. Basic Protocol 1: Isolation of mesenchymal stem cells (MSCs) Support Protocol 1: Characterization of MSCs by flow cytometry Basic Protocol 2: Isolation of neonatal cardiomyoctes (NCMs) Support Protocol 2: Characterization of NCMs Basic Protocol 3: Cardiogenic plasticity of MSCs under ischemic-like conditions Support Protocol 3: Characterization of the cardiomyogenic potential of MSCs.
Keywords: cardiogenic; luciferase; mesenchymal stem cells; neonatal cardiomyocytes.
© 2021 Wiley Periodicals LLC.
Figures
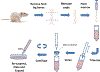



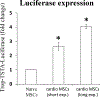
References
-
- Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, . . . Terzic A (2010). Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol, 56(9), 721–734. doi: 10.1016/j.jacc.2010.03.066 - DOI - PMC - PubMed
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, . . . Horwitz E (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317. doi: 10.1080/14653240600855905 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

