CD74 is a regulator of hematopoietic stem cell maintenance
- PMID: 33661886
- PMCID: PMC7963458
- DOI: 10.1371/journal.pbio.3001121
CD74 is a regulator of hematopoietic stem cell maintenance
Abstract
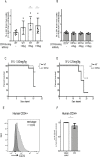
Hematopoietic stem and progenitor cells (HSPCs) are a small population of undifferentiated cells that have the capacity for self-renewal and differentiate into all blood cell lineages. These cells are the most useful cells for clinical transplantations and for regenerative medicine. So far, it has not been possible to expand adult hematopoietic stem cells (HSCs) without losing their self-renewal properties. CD74 is a cell surface receptor for the cytokine macrophage migration inhibitory factor (MIF), and its mRNA is known to be expressed in HSCs. Here, we demonstrate that mice lacking CD74 exhibit an accumulation of HSCs in the bone marrow (BM) due to their increased potential to repopulate and compete for BM niches. Our results suggest that CD74 regulates the maintenance of the HSCs and CD18 expression. Its absence leads to induced survival of these cells and accumulation of quiescent and proliferating cells. Furthermore, in in vitro experiments, blocking of CD74 elevated the numbers of HSPCs. Thus, we suggest that blocking CD74 could lead to improved clinical insight into BM transplant protocols, enabling improved engraftment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



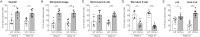



References
-
- Ludin A, Gur-Cohen S, Golan K, Kaufmann KB, Itkin T, Medaglia C, et al. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid Redox Signal. 2014;21(11):1605–19. 10.1089/ars.2014.5941 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

