Neuronal SKN-1B modulates nutritional signalling pathways and mitochondrial networks to control satiety
- PMID: 33661901
- PMCID: PMC7932105
- DOI: 10.1371/journal.pgen.1009358
Neuronal SKN-1B modulates nutritional signalling pathways and mitochondrial networks to control satiety
Abstract
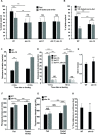
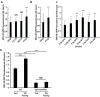
The feeling of hunger or satiety results from integration of the sensory nervous system with other physiological and metabolic cues. This regulates food intake, maintains homeostasis and prevents disease. In C. elegans, chemosensory neurons sense food and relay information to the rest of the animal via hormones to control food-related behaviour and physiology. Here we identify a new component of this system, SKN-1B which acts as a central food-responsive node, ultimately controlling satiety and metabolic homeostasis. SKN-1B, an ortholog of mammalian NF-E2 related transcription factors (Nrfs), has previously been implicated with metabolism, respiration and the increased lifespan incurred by dietary restriction. Here we show that SKN-1B acts in two hypothalamus-like ASI neurons to sense food, communicate nutritional status to the organism, and control satiety and exploratory behaviours. This is achieved by SKN-1B modulating endocrine signalling pathways (IIS and TGF-β), and by promoting a robust mitochondrial network. Our data suggest a food-sensing and satiety role for mammalian Nrf proteins.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- McFarland DJ. Decision making by animals. Nature. 1977. citeulike-article-id:3179069
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

