Incidence and risk factors for major bleeding among patients undergoing percutaneous coronary intervention: Findings from the Norwegian Coronary Stent Trial (NORSTENT)
- PMID: 33661918
- PMCID: PMC7932162
- DOI: 10.1371/journal.pone.0247358
Incidence and risk factors for major bleeding among patients undergoing percutaneous coronary intervention: Findings from the Norwegian Coronary Stent Trial (NORSTENT)
Abstract
Introduction: Bleeding is a concern after percutaneous coronary intervention (PCI) and subsequent dual antiplatelet therapy (DAPT). We herein report the incidence and risk factors for major bleeding in the Norwegian Coronary Stent Trial (NORSTENT).
Materials and methods: NORSTENT was a randomized, double blind, pragmatic trial among patients with acute coronary syndrome or stable coronary disease undergoing PCI during 2008-11. The patients (N = 9,013) were randomized to receive either a drug-eluting stent or a bare-metal stent, and were treated with at least nine months of DAPT. The patients were followed for a median of five years, with Bleeding Academic Research Consortium (BARC) 3-5 major bleeding as one of the safety endpoints. We estimated cumulative incidence of major bleeding by a competing risks model and risk factors through cause-specific Cox models.
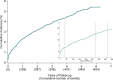
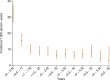
Results: The 12-month cumulative incidence of major bleeding was 2.3%. Independent risk factors for major bleeding were chronic kidney disease, low bodyweight (< 60 kilograms), diabetes mellitus, and advanced age (> 80 years). A myocardial infarction (MI) or PCI during follow-up increased the risk of major bleeding (HR = 1.67, 95% CI 1-29-2.15).
Conclusions: The 12-month cumulative incidence of major bleeding in NORSTENT was higher than reported in previous, explanatory trials. This analysis strengthens the role of chronic kidney disease, advanced age, and low bodyweight as risk factors for major bleeding among patients receiving DAPT after PCI. The presence of diabetes mellitus or recurrent MI among patients is furthermore a signal of increased bleeding risk.
Clinical trial registration: Unique identifier NCT00811772; http://www.clinicaltrial.gov.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al.. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213–60. Epub 2017/09/10. 10.1093/eurheartj/ehx419 . - DOI - PubMed
-
- Costa F, van Klaveren D, James S, Heg D, Raber L, Feres F, et al.. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389(10073):1025–34. 10.1016/S0140-6736(17)30397-5 . - DOI - PubMed
-
- Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, et al.. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315(16):1735–49. Epub 2016/03/30. 10.1001/jama.2016.3775 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

