Physiologically based pharmacokinetic/pharmacodynamic model for the prediction of morphine brain disposition and analgesia in adults and children
- PMID: 33661919
- PMCID: PMC7963108
- DOI: 10.1371/journal.pcbi.1008786
Physiologically based pharmacokinetic/pharmacodynamic model for the prediction of morphine brain disposition and analgesia in adults and children
Abstract
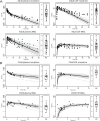
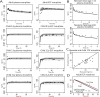
Morphine is a widely used opioid analgesic, which shows large differences in clinical response in children, even when aiming for equivalent plasma drug concentrations. Age-dependent brain disposition of morphine could contribute to this variability, as developmental increase in blood-brain barrier (BBB) P-glycoprotein (Pgp) expression has been reported. In addition, age-related pharmacodynamics might also explain the variability in effect. To assess the influence of these processes on morphine effectiveness, a multi-compartment brain physiologically based pharmacokinetic/pharmacodynamic (PB-PK/PD) model was developed in R (Version 3.6.2). Active Pgp-mediated morphine transport was measured in MDCKII-Pgp cells grown on transwell filters and translated by an in vitro-in vivo extrapolation approach, which included developmental Pgp expression. Passive BBB permeability of morphine and its active metabolite morphine-6-glucuronide (M6G) and their pharmacodynamic parameters were derived from experiments reported in literature. Model simulations after single dose morphine were compared with measured and published concentrations of morphine and M6G in plasma, brain extracellular fluid (ECF) and cerebrospinal fluid (CSF), as well as published drug responses in children (1 day- 16 years) and adults. Visual predictive checks indicated acceptable overlays between simulated and measured morphine and M6G concentration-time profiles and prediction errors were between 1 and -1. Incorporation of active Pgp-mediated BBB transport into the PB-PK/PD model resulted in a 1.3-fold reduced brain exposure in adults, indicating only a modest contribution on brain disposition. Analgesic effect-time profiles could be described reasonably well for older children and adults, but were largely underpredicted for neonates. In summary, an age-appropriate morphine PB-PK/PD model was developed for the prediction of brain pharmacokinetics and analgesic effects. In the neonatal population, pharmacodynamic characteristics, but not brain drug disposition, appear to be altered compared to adults and older children, which may explain the reported differences in analgesic effect.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Wang C, Sadhavisvam S, Krekels EH, Dahan A, Tibboel D, Danhof M, et al.. Developmental changes in morphine clearance across the entire paediatric age range are best described by a bodyweight-dependent exponent model. Clin Drug Investig. 2013;33(7):523–34. Epub 2013/06/12. 10.1007/s40261-013-0097-6 . - DOI - PubMed
-
- Emoto C, Johnson TN, Neuhoff S, Hahn D, Vinks AA, Fukuda T. PBPK Model of Morphine Incorporating Developmental Changes in Hepatic OCT1 and UGT2B7 Proteins to Explain the Variability in Clearances in Neonates and Small Infants. CPT Pharmacometrics Syst Pharmacol. 2018;7(7):464–73. Epub 2018/06/20. 10.1002/psp4.12306 - DOI - PMC - PubMed
-
- Emoto C, Fukuda T, Johnson TN, Neuhoff S, Sadhasivam S, Vinks AA. Characterization of Contributing Factors to Variability in Morphine Clearance Through PBPK Modeling Implemented With OCT1 Transporter. CPT Pharmacometrics Syst Pharmacol. 2017;6(2):110–9. Epub 2016/12/10. 10.1002/psp4.12144 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

