Deleterious mutation accumulation and the long-term fate of chromosomal inversions
- PMID: 33661924
- PMCID: PMC7963061
- DOI: 10.1371/journal.pgen.1009411
Deleterious mutation accumulation and the long-term fate of chromosomal inversions
Abstract
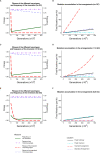
Chromosomal inversions contribute widely to adaptation and speciation, yet they present a unique evolutionary puzzle as both their allelic content and frequency evolve in a feedback loop. In this simulation study, we quantified the role of the allelic content in determining the long-term fate of the inversion. Recessive deleterious mutations accumulated on both arrangements with most of them being private to a given arrangement. This led to increasing overdominance, allowing for the maintenance of the inversion polymorphism and generating strong non-adaptive divergence between arrangements. The accumulation of mutations was mitigated by gene conversion but nevertheless led to the fitness decline of at least one homokaryotype under all considered conditions. Surprisingly, this fitness degradation could be permanently halted by the branching of an arrangement into multiple highly divergent haplotypes. Our results highlight the dynamic features of inversions by showing how the non-adaptive evolution of allelic content can play a major role in the fate of the inversion.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

