Isoalantolactone inhibits pancreatic cancer proliferation by regulation of PI3K and Wnt signal pathway
- PMID: 33661942
- PMCID: PMC7932101
- DOI: 10.1371/journal.pone.0247752
Isoalantolactone inhibits pancreatic cancer proliferation by regulation of PI3K and Wnt signal pathway
Abstract
Background/aims: Isoalantolactone (IATL) is one of multiple isomeric sesquiterpene lactones and is isolated from inula helenium. IATL has multiple functions such as antibacterial, antihelminthic and antiproliferative activities. IATL also inhibits pancreatic cancer proliferation and induces apoptosis by increasing ROS production. However, the detailed mechanism of IATL-mediated pancreatic cancer apoptosis remains largely unknown.
Methods: In current study, pancreatic carcinoma cell lines (PANC-1, AsPC-1, BxPC-3) and a mouse xenograft model were used to determine the mechanism of IATL-mediated toxic effects.
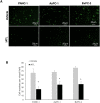
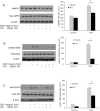
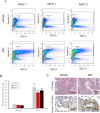
Results: IATL (20μM) inhibited pancreatic adenocarcinoma cell lines proliferation in a time-dependent way; while scratch assay showed that IATL significantly inhibited PANC-1 scratch closure (P<0.05); Invasion assays indicated that IATL significantly attenuated pancreatic adenocarcinoma cell lines invasion on matrigel. Signal analysis showed that IATL inhibited pancreatic adenocarcinoma cell proliferation by blocking EGF-PI3K-Skp2-Akt signal axis. Moreover, IATL induced pancreatic adenocarcinoma cell apoptosis by increasing cytosolic Caspase3 and Box expression. This apoptosis was mediated by inhibition of canonical wnt signal pathway. Finally, xenograft studies showed that IATL also significantly inhibited pancreatic adenocarcinoma cell proliferation and induced pancreatic adenocarcinoma cell apoptosis in vivo.
Conclusions: IATL inhibits pancreatic cancer proliferation and induces apoptosis on cellular and in vivo models. Signal pathway studies reveal that EGF-PI3K-Skp2-Akt signal axis and canonical wnt pathway are involved in IATL-mediated cellular proliferation inhibition and apoptosis. These studies indicate that IATL may provide a future potential therapy for pancreatic cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Rawla P, Thandra KC, Sunkara T. Pancreatic cancer and obesity: epidemiology, mechanism, and preventive strategies. Clin J Gastroenterol. 2019. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

