Validation of a new automated chemiluminescent anti-SARS-CoV-2 IgM and IgG antibody assay system detecting both N and S proteins in Japan
- PMID: 33661990
- PMCID: PMC7932516
- DOI: 10.1371/journal.pone.0247711
Validation of a new automated chemiluminescent anti-SARS-CoV-2 IgM and IgG antibody assay system detecting both N and S proteins in Japan
Abstract
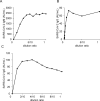
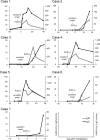
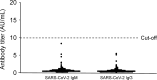
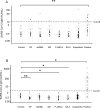
PCR methods are presently the standard for the diagnosis of Coronavirus disease 2019 (COVID-19), but additional methodologies are needed to complement PCR methods, which have some limitations. Here, we validated and investigated the usefulness of measuring serum antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using the iFlash3000 CLIA analyzer. We measured IgM and IgG titers against SARS-CoV-2 in sera collected from 26 PCR-positive COVID-19 patients, 53 COVID-19-suspected but PCR-negative patients, and 20 and 100 randomly selected non-COVID-19 patients who visited our hospital in 2020 and 2017, respectively. The repeatability and within-laboratory precision were obviously good in validations, following to the CLSI document EP15-A3. Linearity was also considered good between 0.6 AU/mL and 112.7 AU/mL for SARS-CoV-2 IgM and between 3.2 AU/mL and 55.3 AU/mL for SARS-CoV-2 IgG, while the linearity curves plateaued above the upper measurement range. We also confirmed that the seroconversion and no-antibody titers were over the cutoff values in all 100 serum samples collected in 2017. These results indicate that this measurement system successfully detects SARS-CoV-2 IgM/IgG. We observed four false-positive cases in the IgM assay and no false-positive cases in the IgG assay when 111 serum samples known to contain autoantibodies were evaluated. The concordance rates of the antibody test with the PCR test were 98.1% for SARS-CoV-2 IgM and 100% for IgG among PCR-negative cases and 30.8% for SARS-CoV-2 IgM and 73.1% for SARS-CoV-2 IgG among PCR-positive cases. In conclusion, the performance of this new automated method for detecting antibody against both N and S proteins of SARS-CoV-2 is sufficient for use in laboratory testing.
Conflict of interest statement
The present study is a collaborative research project among The University of Tokyo, Shenzhen YHLO Biotech Co., Ltd, and Medical & Biological Laboratories Co., Ltd. F. X. and F. H. are employees of Shenzhen YHLO Biotech Co., Ltd and Y. K. and J. O. are employees of Medical & Biological Laboratories Co., Ltd. Other authors received no consultancy or patents from these companies. The SARS-CoV-2 IgM and IgG CLIA kits, which detect N and S proteins, were marketed products and the SARS-CoV-2 IgM and IgG CLIA kits, which detect N or S proteins separately, were the products in development. These conflicts of interest do not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

