Effect of Yttrium-90 transarterial radioembolization in patients with non-surgical hepatocellular carcinoma: A systematic review and meta-analysis
- PMID: 33662011
- PMCID: PMC7932100
- DOI: 10.1371/journal.pone.0247958
Effect of Yttrium-90 transarterial radioembolization in patients with non-surgical hepatocellular carcinoma: A systematic review and meta-analysis
Abstract
Background: Recently, the use of Yttrium-90 transarterial radioembolization in non-surgical hepatocellular carcinoma was suggested but the evidence supporting its use is unclear.
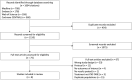
Methods: We searched Medline, Embase, Web of Science and Cochrane CENTRAL from inception up to April 14, 2020 for randomized controlled trials comparing Y90-TARE to standard of care in non-surgical HCC patients. Our primary outcome was overall survival (OS). Our secondary outcomes were progression-free survival, time to progression, disease control rate, grade ≥3 adverse events and rates of gastro-intestinal ulcers. Hazard ratios (HR) and risk ratios (RR) with random-effects model were used for our analyses. The risk of bias of the included studies was assessed using Cochrane's RoB 2 tool.
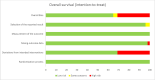
Results: Of 1,604 citations identified, eight studies (1,439 patients) were included in our analysis. No improvement in overall survival were noted when Yttrium-90 transarterial radioembolization was compared to standard treatments (HR 0.99 [95% CI 0.81-1.21], 6 studies, I2 = 77.6%). However, Yttrium-90 transarterial radioembolization was associated with fewer grade ≥3 adverse events (RR 0.64 [95% CI 0.45-0.92], 7 studies, I2 = 66%). No difference was observed on other secondary outcomes.
Discussion: In non-surgical HCC patients, Yttrium-90 transarterial radioembolization was not associated with significant effect on survival, progression-free survival, time to progression, disease control rate and the incidence of gastro-intestinal ulcers but was however associated with significantly lower rates of grade ≥3 adverse events. Further randomized controlled trials are warranted to better delineate optimal treatment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Global Burden of Disease Liver Cancer C, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, et al.. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3(12):1683–91. Epub 2017/10/07. 10.1001/jamaoncol.2017.3055 - DOI - PMC - PubMed
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al.. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. Epub 2008/12/20. 10.1016/S1470-2045(08)70285-7 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

