SARS-CoV-2 on Ocular Surfaces in a Cohort of Patients With COVID-19 From the Lombardy Region, Italy
- PMID: 33662099
- PMCID: PMC7934077
- DOI: 10.1001/jamaophthalmol.2020.5464
SARS-CoV-2 on Ocular Surfaces in a Cohort of Patients With COVID-19 From the Lombardy Region, Italy
Abstract
Importance: Since February 2020, coronavirus disease 2019 (COVID-19) has spread rapidly all over the world, with an epidemiological cluster in Lombardy, Italy. The viral communicability may be mediated by various body fluids, but insufficient information is available on the presence of the virus in human tears.
Objectives: To investigate the rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in tears collected from patients with COVID-19 by means of real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) assay and to assess the association of virus presence with concomitant clinical conditions.
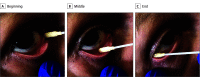
Design, setting, and participants: Cross-sectional study conducted between April 9 and May 5, 2020. The setting was intensive care units at Azienda Socio-Sanitaria Territoriale (ASST) Sette-Laghi Hospital, University of Insubria, in Varese, Lombardy, Italy. A conjunctival swab was performed in 91 patients hospitalized for COVID-19, which was clinically diagnosed by rRT-PCR assay on nasopharyngeal swabs and by radiological imaging. Conjunctival swabs from 17 additional healthy volunteer participants with no symptoms of COVID-19 were examined to evaluate the availability and applicability of the conjunctival swab test.
Exposure: SARS-CoV-2 detection by means of rRT-PCR assay performed on the collected samples obtained by conjunctival swabs.
Main outcomes and measures: Conjunctival swab and nasopharyngeal swab results are reported, as well as demographic and clinical data.
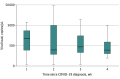
Results: A total of 108 participants (mean [SD] age, 58.7 [14.2] years; 55 female and 53 male) were tested for SARS-CoV-2 using rRT-PCR assay, including 91 patients hospitalized with COVID-19 and 17 were healthy volunteers. SARS-CoV-2 was found on the ocular surface in 52 of 91 patients with COVID-19 (57.1%; 95% CI, 46.3%-67.5%), with a wide variability in the mean viral load from both eyes. Among a subset of 41 patients, concordance of 63.0% (95% CI, 41.0%-81.0%) was found between positive conjunctival and nasopharyngeal swab test results when performed within 2 days of each other. In 17 of these patients, nasopharyngeal swab results were negative for SARS-CoV-2. In 10 of these 17 patients, conjunctival swab results were positive for the virus.
Conclusions and relevance: In this study, SARS-CoV-2 RNA was found on the ocular surface in a large part of this cohort of patients with COVID-19, although the infectivity of this material could not be determined. Because patients may have positive test results with a conjunctival swab and negative results with a nasopharyngeal swab, use of the slightly invasive conjunctival swab may be considered as a supplementary diagnostic test.
Conflict of interest statement
Figures
Comment in
-
Live and Replication-Competent SARS-CoV-2 in Ocular Fluids.JAMA Ophthalmol. 2021 Sep 1;139(9):1041. doi: 10.1001/jamaophthalmol.2021.2681. JAMA Ophthalmol. 2021. PMID: 34323930 No abstract available.
-
Live and Replication-Competent SARS-CoV-2 in Ocular Fluids-Reply.JAMA Ophthalmol. 2021 Sep 1;139(9):1041-1042. doi: 10.1001/jamaophthalmol.2021.2751. JAMA Ophthalmol. 2021. PMID: 34323936 No abstract available.
Similar articles
-
SARS-CoV-2 and the ocular surface: test accuracy and viral load.Arq Bras Oftalmol. 2023 Apr 3;87(5):e20220172. doi: 10.5935/0004-2749.2022-0172. eCollection 2023. Arq Bras Oftalmol. 2023. PMID: 39298730 Free PMC article.
-
Evaluation of SARS-CoV-2 in Tears of Patients with Moderate to Severe COVID-19.Ophthalmology. 2021 Apr;128(4):494-503. doi: 10.1016/j.ophtha.2020.08.029. Epub 2020 Aug 31. Ophthalmology. 2021. PMID: 32882309 Free PMC article.
-
No secret hiding place? Absence of SARS-CoV-2 on the ocular surface of 1145 hospitalized patients in a pandemic area.Graefes Arch Clin Exp Ophthalmol. 2021 Jun;259(6):1605-1608. doi: 10.1007/s00417-021-05086-3. Epub 2021 Jan 29. Graefes Arch Clin Exp Ophthalmol. 2021. PMID: 33511435 Free PMC article.
-
Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis.J Med Virol. 2021 Feb;93(2):719-725. doi: 10.1002/jmv.26349. Epub 2020 Aug 2. J Med Virol. 2021. PMID: 32706393 Free PMC article.
-
Conjunctivitis as sole symptom of COVID-19: A case report and review of literature.Eur J Ophthalmol. 2021 Mar;31(2):NP161-NP166. doi: 10.1177/1120672120946287. Epub 2020 Jul 24. Eur J Ophthalmol. 2021. PMID: 32703010 Free PMC article. Review.
Cited by
-
Changes in the ocular surface microbiome of patients with coronavirus disease 2019 (COVID-19).Front Microbiol. 2024 Jul 8;15:1389139. doi: 10.3389/fmicb.2024.1389139. eCollection 2024. Front Microbiol. 2024. PMID: 39040901 Free PMC article.
-
COVID-19 pandemic decreased the ophthalmic outpatient numbers and altered the diagnosis distribution in a community hospital in Taiwan: An observational study.PLoS One. 2022 Mar 8;17(3):e0264976. doi: 10.1371/journal.pone.0264976. eCollection 2022. PLoS One. 2022. PMID: 35259188 Free PMC article.
-
Safety and Efficacy of Cataract Surgery Under a Local Infection Control Protocol Before and During a COVID-19 Wave in Thailand for Healthcare Workers and Patients: A Prospective Cohort from a Secondary Center.Clin Ophthalmol. 2022 Jun 3;16:1773-1781. doi: 10.2147/OPTH.S366353. eCollection 2022. Clin Ophthalmol. 2022. PMID: 35685377 Free PMC article.
-
Macrophage Activation in Follicular Conjunctivitis during the COVID-19 Pandemic.Microorganisms. 2023 Aug 31;11(9):2198. doi: 10.3390/microorganisms11092198. Microorganisms. 2023. PMID: 37764042 Free PMC article.
-
SARS-CoV-2-induced sensory perturbations: A narrative review of clinical phenotypes, molecular pathologies, and possible interventions.Brain Behav Immun Health. 2025 Mar 24;45:100983. doi: 10.1016/j.bbih.2025.100983. eCollection 2025 May. Brain Behav Immun Health. 2025. PMID: 40231214 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

