Oxidation-Induced "One-Pot" Click Chemistry
- PMID: 33662210
- PMCID: PMC8227495
- DOI: 10.1021/acs.chemrev.0c01180
Oxidation-Induced "One-Pot" Click Chemistry
Abstract
Click chemistry has been established rapidly as one of the most valuable methods for the chemical transformation of complex molecules. Due to the rapid rates, clean conversions to the products, and compatibility of the reagents and reaction conditions even in complex settings, it has found applications in many molecule-oriented disciplines. From the vast landscape of click reactions, approaches have emerged in the past decade centered around oxidative processes to generate in situ highly reactive synthons from dormant functionalities. These approaches have led to some of the fastest click reactions know to date. Here, we review the various methods that can be used for such oxidation-induced "one-pot" click chemistry for the transformation of small molecules, materials, and biomolecules. A comprehensive overview is provided of oxidation conditions that induce a click reaction, and oxidation conditions are orthogonal to other click reactions so that sequential "click-oxidation-click" derivatization of molecules can be performed in one pot. Our review of the relevant literature shows that this strategy is emerging as a powerful approach for the preparation of high-performance materials and the generation of complex biomolecules. As such, we expect that oxidation-induced "one-pot" click chemistry will widen in scope substantially in the forthcoming years.
Conflict of interest statement
The authors declare the following competing financial interest(s): FVD is an employee of Synaffix BV.
Figures


















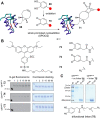
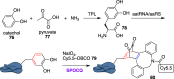







References
-
- Kalgutkar A. S.; Gardner I.; Obach R. S.; Shaffer C. L.; Callegari E.; Henne K. R.; Mutlib A. E.; Dalvie D. K.; Lee J. S.; Nakari Y.; O’Donnell J. P.; Boer J.; Harriman S. P. A comprehensive listing of bioactivation pathways of organic functional groups. Curr. Drug Metab. 2005, 6, 161–225. 10.2174/1389200054021799. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

