Recombinant ADAMTS13 reduces abnormally up-regulated von Willebrand factor in plasma from patients with severe COVID-19
- PMID: 33662796
- PMCID: PMC7890348
- DOI: 10.1016/j.thromres.2021.02.012
Recombinant ADAMTS13 reduces abnormally up-regulated von Willebrand factor in plasma from patients with severe COVID-19
Abstract
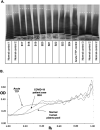
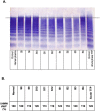
Thrombosis affecting the pulmonary and systemic vasculature is common during severe COVID-19 and causes adverse outcomes. Although thrombosis likely results from inflammatory activation of vascular cells, the mediators of thrombosis remain unconfirmed. In a cross-sectional cohort of 36 severe COVID-19 patients, we show that markedly increased plasma von Willebrand factor (VWF) levels were accompanied by a partial reduction in the VWF regulatory protease ADAMTS13. In all patients we find this VWF/ADAMTS13 imbalance to be associated with persistence of ultra-high-molecular-weight (UHMW) VWF multimers that are highly thrombogenic in some disease settings. Incubation of plasma samples from patients with severe COVID-19 with recombinant ADAMTS13 (rADAMTS13) substantially reduced the abnormally high VWF activity, reduced overall multimer size and depleted UHMW VWF multimers in a time and concentration dependent manner. Our data implicate disruption of normal VWF/ADAMTS13 homeostasis in the pathogenesis of severe COVID-19 and indicate that this can be reversed ex vivo by correction of low plasma ADAMTS13 levels. These findings suggest a potential therapeutic role for rADAMTS13 in helping restore haemostatic balance in COVID-19 patients.
Keywords: ADAMTS13; COVID-19; Endothelium; Inflammation; SARS-COV-2; Thrombosis; rADAMTS13; von Willebrand factor.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
PLT, GS, HG, BE, BM, WE, and NJ are employees of subsidiaries of Takeda pharmaceutical company limited and are Takeda stock owners. ML and SR have received funding support from Takeda. NBB is an employee of Technoclone. AM, RP, CRS, RD, RK and AD declare no relevant conflicts of interest.
Figures





References
-
- Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J., Dela Cruz C.S., Dumont A., Halene S., Hwa J., Koff J., Menninger H., Neparidze N., Price C., Siner J.M., Tormey C., Rinder H.M., Chun H.J., Lee A.I. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. - DOI - PMC - PubMed
-
- Wichmann D., Sperhake J.P., Lutgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schroder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Puschel K., Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020;73(4):268–277. doi: 10.7326/M20-2003. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

