Cerebral amyloid-β load is associated with neurodegeneration and gliosis: Mediation by p-tau and interactions with risk factors early in the Alzheimer's continuum
- PMID: 33663013
- PMCID: PMC8252618
- DOI: 10.1002/alz.12245
Cerebral amyloid-β load is associated with neurodegeneration and gliosis: Mediation by p-tau and interactions with risk factors early in the Alzheimer's continuum
Abstract
Introduction: The association between cerebral amyloid-β accumulation and downstream CSF biomarkers is not fully understood, particularly in asymptomatic stages.
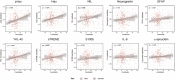
Methods: In 318 cognitively unimpaired participants, we assessed the association between amyloid-β PET (Centiloid), and cerebrospinal fluid (CSF) biomarkers of several pathophysiological pathways. Interactions by Alzheimer's disease risk factors (age, sex and APOE-ε4), and the mediation effect of tau and neurodegeneration were also investigated.
Results: Centiloids were positively associated with CSF biomarkers of tau pathology (p-tau), neurodegeneration (t-tau, NfL), synaptic dysfunction (neurogranin) and neuroinflammation (YKL-40, GFAP, sTREM2), presenting interactions with age (p-tau, t-tau, neurogranin) and sex (sTREM2, NfL). Most of these associations were mediated by p-tau, except for NfL. The interaction between sex and amyloid-β on sTREM2 and NfL was also tau-independent.
Discussion: Early amyloid-β accumulation has a tau-independent effect on neurodegeneration and a tau-dependent effect on neuroinflammation. Besides, sex has a modifier effect on these associations independent of tau.
Keywords: Alzheimer; [18F]flutemetamol; biomarkers; glial activation; inflammation; modulation; neuronal injury; preclinical.
© 2021 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
JLM is currently a full time employee of H. Lundbeck A/S and priory has served as a consultant or at advisory boards for the following for‐profit companies, or has given lectures in symposia sponsored by the following for‐profit companies: Roche Diagnostics, Genentech, Novartis, Lundbeck, Oryzon, Biogen, Lilly, Janssen, Green Valley, MSD, Eisai, Alector, BioCross, GE Healthcare, ProMIS Neurosciences. HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. GK is a full time employee of Roche Diagnostics GmbH. The remaining authors declare that they have no conflict of interest.
Figures


References
-
- Molinuevo JL, Ayton S, Batrla R, Bednar MM, Bittner T, Cummings J, Fagan AM, Hampel H, Mielke MM, Mikulskis A, O’Bryant S, Scheltens P, Sevigny J, Shaw LM, Soares HD, Tong G, Trojanowski JQ, Zetterberg H, Blennow K. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathologica 2018;136(6):821‐853. 10.1007/s00401-018-1932-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

