Health assessment of wild speckled dwarf tortoises, CHERSOBIUS SIGNATUS
- PMID: 33663511
- PMCID: PMC7934230
- DOI: 10.1186/s12917-021-02800-5
Health assessment of wild speckled dwarf tortoises, CHERSOBIUS SIGNATUS
Abstract
Background: In free-ranging reptile populations, bacterial, fungal, viral and parasitic pathogens may affect hosts through impairment in movements, thermoregulation, reproduction, survival, and population dynamics. The speckled dwarf tortoise (Chersobius [Homopus] signatus) is a threatened species that is mostly restricted to the Succulent Karoo biome in South Africa, and little information on pathogens of this species is available yet. We derived baseline parameters for five males and five females that were captured to genetically enhance a conservation breeding program in Europe. Upon collection of the tortoises, ticks were removed and identified. Immediately upon arrival in Europe, ocular, nasal, oral and cloacal swabs were taken for viral, bacteriological and mycological examinations. Fecal samples were collected before and 1 month after fenbendazole treatment, and analyzed for parasites. A panel of PCR, aiming to detect herpesviruses, adenoviruses and iridoviruses, was carried out.
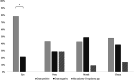
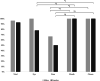
Results: Samples were negative for viruses, while bacteriological examination yielded detectable growth in 82.5% of the swabs with a mean load of 16 × 107 ± 61 × 108 colony forming units (CFU) per swab, representing 34 bacterial species. Cloacal and oral swabs yielded higher detectable growth loads than nasal and ocular swabs, but no differences between sexes were observed. Fungi and yeasts (mean load 5 × 103 ± 13 × 103 CFU/swab) were detected in 25% of the swabs. All pre-treatment fecal samples were positive for oxyurid eggs, ranging from 200 to 2400 eggs per gram of feces, whereas after the treatment a significantly reduced egg count (90-100% reduction) was found in seven out of 10 individuals. One remaining individual showed 29% reduction, and two others had increased egg counts. In five tortoises, Nycthocterus spp. and coccidian oocysts were also identified. Soft ticks were identified as Ornithodoros savignyi.
Conclusions: Our baseline data from clinically healthy individuals will help future studies to interpret prevalences of microorganisms in speckled dwarf tortoise populations. The study population did not appear immediately threatened by current parasite presence.
Keywords: Chersobius [Homopus] signatus; Health assessment; Reptile; Tortoise; Wildlife.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Jacobson ER. Infectious diseases and pathology of reptiles. Boca Raton, Florida, USA: CRC Press; 2007.
-
- Fournié G, Goodman SJ, Cruz M, Cede V, Vélez A, Pati L, Millins C, Gibbons LM, Fox MT, Cunning AA. Biogeography of parasitic nematode communities in the Galápagos Giant tortoise: implications for conservation management. PLoS One. 2015;10(9):e0135684. doi: 10.1371/journal.pone.0135684. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

