Tip-to-Base LAMPOON for Transcatheter Mitral Valve Replacement With a Protected Mitral Annulus
- PMID: 33663781
- PMCID: PMC8080374
- DOI: 10.1016/j.jcin.2020.11.034
Tip-to-Base LAMPOON for Transcatheter Mitral Valve Replacement With a Protected Mitral Annulus
Erratum in
-
Correction.JACC Cardiovasc Interv. 2021 Oct 11;14(19):2194. doi: 10.1016/j.jcin.2021.08.016. JACC Cardiovasc Interv. 2021. PMID: 34620401 No abstract available.
Abstract
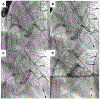
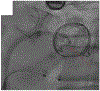
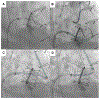
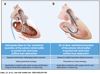
Objectives: The purpose of this study was to evaluate tip-to-base intentional laceration of the anterior mitral leaflet to prevent left ventricular outflow tract obstruction (LAMPOON) in patients undergoing transcatheter mitral valve replacement (TMVR) in annuloplasty rings or surgical mitral valves.
Background: LAMPOON is an effective adjunct to TMVR that prevents left ventricular outflow tract obstruction (LVOTO). Laceration is typically performed from the base to the tip of the anterior mitral leaflet. A modified laceration technique from leaflet tip to base may be effective in patients with a prosthesis that protects the aortomitral curtain.
Methods: This is a multicenter, 21-patient, consecutive retrospective observational cohort. Patients underwent tip-to-base LAMPOON to prevent LVOTO and leaflet overhang, or therapeutically to lacerate a long anterior mitral leaflet risking or causing LVOTO. Outcomes were compared with findings from patients in the LAMPOON investigational device exemption trial with a prior mitral annuloplasty.
Results: Twenty-one patients with a annuloplasty or valve prosthesis-protected mitral annulus underwent tip-to-base LAMPOON (19 preventive, 2 rescue). Leaflet laceration was successful in all and successfully prevented or treated LVOTO in all patients. No patients had significant LVOTO upon discharge. There were 2 cases of unintentional aortic valve injury (1 patient underwent emergency transcatheter aortic valve replacement and 1 patient underwent urgent surgical aortic valve replacement). In both cases, the patients had a supra-annular ring annuloplasty, and the retrograde aortic guiding catheter failed to insulate the guidewire lacerating surface from the aortic root. All patients survived to 30 days. Compared with classic retrograde LAMPOON, there was a trend toward shorter procedure time.
Conclusions: Tip-to-base laceration is a simple, effective, and safe LAMPOON variant applicable to patients with an appropriately positioned mitral annular ring or bioprosthetic valve. Operators should take care to insulate the lacerating surface from adjacent structures.
Keywords: left ventricular outflow tract obstruction; mitral annuloplasty; mitral regurgitation; mitral stenosis; surgical mitral valve replacement; transcatheter electrosurgery; transcatheter mitral valve replacement.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Funding Support and Author Disclosures This work was supported by the Emory Structural Heart and Valve program intramural funds and by National Institutes of Health Grant No. Z01-HL006040. Dr. Lisko’s employer has contracts for BASILICA analysis with Medtronic and Edwards Lifesciences. Dr. Babaliaros has served as a consultant for Edwards Lifesciences and Abbott Vascular; has an employer with research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific; and owns equity interest in Transmural Systems. Drs. Khan and Rogers have served as a proctor for Edwards Lifesciences and Medtronic. Drs. Khan, Lederman, and Rogers are co-inventors on patents, assigned to the National Institutes of Health, on devices for electrosurgical leaflet laceration. Dr. Paone has served as a consultant and proctor for Edwards Lifesciences. Dr. McCabe has served as consultant for Edwards Lifesciences, Boston Scientific, and Teleflex. Dr. Grubb has served as a speaker, proctor, and principal investigator for Edwards Lifesciences; served as a speaker, proctor, and advisory board member for Boston Scientific; and served as a speaker, proctor, principal investigator, advisory board member, and national principal investigator for Medtronic. Dr. Lederman has served as the principal investigator on a cooperative research and development agreement between National Institutes of Health and Edwards Lifesciences for transcatheter modification of the mitral valve. Dr. Greenbaum has served as a proctor for Edwards Lifesciences, Medtronic, and Abbott Vascular; owns an equity interest in Transmural Systems; and has an employer with research contracts for clinical investigation of transcatheter aortic, mitral, and tricuspid devices from Edwards Lifesciences, Abbott Vascular, Medtronic, and Boston Scientific. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
Comment in
-
The Multiple Faces of LAMPOON.JACC Cardiovasc Interv. 2021 Mar 8;14(5):551-553. doi: 10.1016/j.jcin.2020.12.031. Epub 2021 Mar 1. JACC Cardiovasc Interv. 2021. PMID: 33663782 No abstract available.
References
-
- Guerrero M, Dvir D, Himbert D, et al. Transcatheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification: results from the first multicenter global registry. J Am Coll Cardiol Intv 2016;9:1361–71. - PubMed
-
- Guerrero M, Urena M, Himbert D, et al. 1-Year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol 2018;71:1841–53. - PubMed
-
- Yoon SH, Bleiziffer S, Latib A, et al. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. J Am Coll Cardiol Intv 2019;12:182–93. - PubMed
-
- Yoon SH, Whisenant BK, Bleiziffer S, et al. Transcatheter mitral valve replacement for degenerated bioprosthetic valves and failed annuloplasty rings. J Am Coll Cardiol 2017;70:1121–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

