Synaptophysin Regulates Fusion Pores and Exocytosis Mode in Chromaffin Cells
- PMID: 33664131
- PMCID: PMC8055083
- DOI: 10.1523/JNEUROSCI.2833-20.2021
Synaptophysin Regulates Fusion Pores and Exocytosis Mode in Chromaffin Cells
Abstract
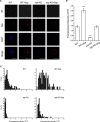
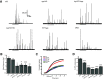
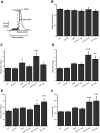
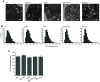
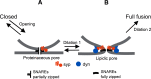
Synaptophysin (syp) is a major integral membrane protein of secretory vesicles. Previous work has demonstrated functions for syp in synaptic vesicle cycling, endocytosis, and synaptic plasticity, but the role of syp in the process of membrane fusion during Ca2+-triggered exocytosis remains poorly understood. Furthermore, although syp resides on both large dense-core and small synaptic vesicles, its role in dense-core vesicle function has received less attention compared with synaptic vesicle function. To explore the role of syp in membrane fusion and dense-core vesicle function, we used amperometry to measure catecholamine release from single vesicles in male and female mouse chromaffin cells with altered levels of syp and the related tetraspanner protein synaptogyrin (syg). Knocking out syp slightly reduced the frequency of vesicle fusion events below wild-type (WT) levels, but knocking out both syp and syg reduced the frequency 2-fold. Knocking out both proteins stabilized initial fusion pores, promoted fusion pore closure (kiss-and-run), and reduced late-stage fusion pore expansion. Introduction of a syp construct lacking its C-terminal dynamin-binding domain in syp knock-outs (KOs) increased the duration and fraction of kiss-and-run events, increased total catecholamine release per event, and reduced late-stage fusion pore expansion. These results demonstrated that syp and syg regulate dense-core vesicle function at multiple stages to initiate fusion, control the choice of mode between full-fusion and kiss-and-run, and influence the dynamics of both initial and late-stage fusion pores. The transmembrane domain (TMD) influences small initial fusion pores, and the C-terminal domain influences large late-stage fusion pores, possibly through an interaction with dynamin.SIGNIFICANCE STATEMENT The secretory vesicle protein synaptophysin (syp) is known to function in synaptic vesicle cycling, but its roles in dense-core vesicle functions, and in controlling membrane fusion during Ca2+-triggered exocytosis remain unclear. The present study used amperometry recording of catecholamine release from endocrine cells to assess the impact of syp and related proteins on membrane fusion. A detailed analysis of amperometric spikes arising from the exocytosis of single vesicles showed that these proteins influence fusion pores at multiple stages and control the choice between kiss-and-run and full-fusion. Experiments with a syp construct lacking its C terminus indicated that the transmembrane domain (TMD) influences the initial fusion pore, while the C-terminal domain influences later stages after fusion pore expansion.
Keywords: chromaffin cells; dense-core vesicle; exocytosis; fusion pores; secretion; synaptophysin.
Copyright © 2021 the authors.
Figures






Similar articles
-
Synaptophysin transmembrane domain III controls fusion pore dynamics in Ca2+-triggered exocytosis.Biophys J. 2023 Jun 6;122(11):1962-1973. doi: 10.1016/j.bpj.2022.09.029. Epub 2022 Sep 27. Biophys J. 2023. PMID: 36168290 Free PMC article.
-
Full-fusion and kiss-and-run in chromaffin cells controlled by irreversible vesicle size-dependent fusion pore transitions.Cell Calcium. 2022 Jul;105:102606. doi: 10.1016/j.ceca.2022.102606. Epub 2022 May 21. Cell Calcium. 2022. PMID: 35636152
-
The Phosphoprotein Synapsin Ia Regulates the Kinetics of Dense-Core Vesicle Release.J Neurosci. 2021 Mar 31;41(13):2828-2841. doi: 10.1523/JNEUROSCI.2593-19.2021. Epub 2021 Feb 25. J Neurosci. 2021. PMID: 33632727 Free PMC article.
-
Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis?Bioessays. 2004 Apr;26(4):445-53. doi: 10.1002/bies.20012. Bioessays. 2004. PMID: 15057942 Review.
-
Disruption of Exocytosis in Sympathoadrenal Chromaffin Cells from Mouse Models of Neurodegenerative Diseases.Int J Mol Sci. 2020 Mar 12;21(6):1946. doi: 10.3390/ijms21061946. Int J Mol Sci. 2020. PMID: 32178443 Free PMC article. Review.
Cited by
-
Endoplasmic Reticulum-Plasma Membrane Junctions as Sites of Depolarization-Induced Ca2+ Signaling in Excitable Cells.Annu Rev Physiol. 2023 Feb 10;85:217-243. doi: 10.1146/annurev-physiol-032122-104610. Epub 2022 Oct 6. Annu Rev Physiol. 2023. PMID: 36202100 Free PMC article. Review.
-
Bridging the gap between presynaptic hair cell function and neural sound encoding.Elife. 2024 Dec 24;12:RP93749. doi: 10.7554/eLife.93749. Elife. 2024. PMID: 39718472 Free PMC article.
-
Synaptophysin transmembrane domain III controls fusion pore dynamics in Ca2+-triggered exocytosis.Biophys J. 2023 Jun 6;122(11):1962-1973. doi: 10.1016/j.bpj.2022.09.029. Epub 2022 Sep 27. Biophys J. 2023. PMID: 36168290 Free PMC article.
-
Fresh Gastrodia elata Blume alleviates simulated weightlessness-induced cognitive impairment by regulating inflammatory and apoptosis-related pathways.Front Pharmacol. 2023 May 3;14:1173920. doi: 10.3389/fphar.2023.1173920. eCollection 2023. Front Pharmacol. 2023. PMID: 37205911 Free PMC article.
-
Acute stress response on Atlantic salmon: a time-course study of the effects on plasma metabolites, mucus cortisol levels, and head kidney transcriptome profile.Fish Physiol Biochem. 2023 Feb;49(1):97-116. doi: 10.1007/s10695-022-01163-4. Epub 2022 Dec 27. Fish Physiol Biochem. 2023. PMID: 36574113 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
