Glutamatergic Neurons in the Preoptic Hypothalamus Promote Wakefulness, Destabilize NREM Sleep, Suppress REM Sleep, and Regulate Cortical Dynamics
- PMID: 33664133
- PMCID: PMC8051693
- DOI: 10.1523/JNEUROSCI.2718-20.2021
Glutamatergic Neurons in the Preoptic Hypothalamus Promote Wakefulness, Destabilize NREM Sleep, Suppress REM Sleep, and Regulate Cortical Dynamics
Abstract
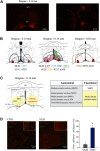
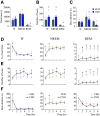
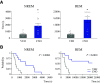
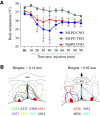
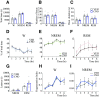
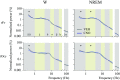
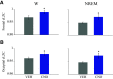
Clinical and experimental data from the last nine decades indicate that the preoptic area of the hypothalamus is a critical node in a brain network that controls sleep onset and homeostasis. By contrast, we recently reported that a group of glutamatergic neurons in the lateral and medial preoptic area increases wakefulness, challenging the long-standing notion in sleep neurobiology that the preoptic area is exclusively somnogenic. However, the precise role of these subcortical neurons in the control of behavioral state transitions and cortical dynamics remains unknown. Therefore, in this study, we used conditional expression of excitatory hM3Dq receptors in these preoptic glutamatergic (Vglut2+) neurons and show that their activation initiates wakefulness, decreases non-rapid eye movement (NREM) sleep, and causes a persistent suppression of rapid eye movement (REM) sleep. We also demonstrate, for the first time, that activation of these preoptic glutamatergic neurons causes a high degree of NREM sleep fragmentation, promotes state instability with frequent arousals from sleep, decreases body temperature, and shifts cortical dynamics (including oscillations, connectivity, and complexity) to a more wake-like state. We conclude that a subset of preoptic glutamatergic neurons can initiate, but not maintain, arousals from sleep, and their inactivation may be required for NREM stability and REM sleep generation. Further, these data provide novel empirical evidence supporting the hypothesis that the preoptic area causally contributes to the regulation of both sleep and wakefulness.SIGNIFICANCE STATEMENT Historically, the preoptic area of the hypothalamus has been considered a key site for sleep generation. However, emerging modeling and empirical data suggest that this region might play a dual role in sleep-wake control. We demonstrate that chemogenetic stimulation of preoptic glutamatergic neurons produces brief arousals that fragment sleep, persistently suppresses REM sleep, causes hypothermia, and shifts EEG patterns toward a "lighter" NREM sleep state. We propose that preoptic glutamatergic neurons can initiate, but not maintain, arousal from sleep and gate REM sleep generation, possibly to block REM-like intrusions during NREM-to-wake transitions. In contrast to the long-standing notion in sleep neurobiology that the preoptic area is exclusively somnogenic, we provide further evidence that preoptic neurons also generate wakefulness.
Keywords: DREADDs; arousal; consciousness; gamma; sleep fragmentation; slow oscillations.
Copyright © 2021 Mondino et al.
Figures




Similar articles
-
Regulation of REM and NREM Sleep by Preoptic Glutamatergic Neurons.Sleep. 2025 May 26:zsaf141. doi: 10.1093/sleep/zsaf141. Online ahead of print. Sleep. 2025. PMID: 40417998
-
Cholinergic, Glutamatergic, and GABAergic Neurons of the Pedunculopontine Tegmental Nucleus Have Distinct Effects on Sleep/Wake Behavior in Mice.J Neurosci. 2017 Feb 1;37(5):1352-1366. doi: 10.1523/JNEUROSCI.1405-16.2016. Epub 2016 Dec 30. J Neurosci. 2017. PMID: 28039375 Free PMC article.
-
[Neurochemical mechanisms of sleep regulation].Glas Srp Akad Nauka Med. 2009;(50):97-109. Glas Srp Akad Nauka Med. 2009. PMID: 20666118 Review. Serbian.
-
NMDA Receptors in the Lateral Preoptic Hypothalamus Are Essential for Sustaining NREM and REM Sleep.J Neurosci. 2022 Jul 6;42(27):5389-5409. doi: 10.1523/JNEUROSCI.0350-21.2022. Epub 2022 Jun 1. J Neurosci. 2022. PMID: 35649726 Free PMC article.
-
Control of sleep and wakefulness.Physiol Rev. 2012 Jul;92(3):1087-187. doi: 10.1152/physrev.00032.2011. Physiol Rev. 2012. PMID: 22811426 Free PMC article. Review.
Cited by
-
Elevated peripheral glutamate and upregulated expression of NMDA receptor NR1 subunit in insomnia disorder.Front Psychiatry. 2024 Oct 7;15:1436024. doi: 10.3389/fpsyt.2024.1436024. eCollection 2024. Front Psychiatry. 2024. PMID: 39435127 Free PMC article.
-
Sleep Disorders: Pathogenesis and Therapeutic Interventions.MedComm (2020). 2025 Mar 10;6(3):e70130. doi: 10.1002/mco2.70130. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 40066230 Free PMC article. Review.
-
Effects of clozapine-N-oxide and compound 21 on sleep in laboratory mice.Elife. 2023 Mar 9;12:e84740. doi: 10.7554/eLife.84740. Elife. 2023. PMID: 36892930 Free PMC article.
-
Circadian Rhythms Disrupted by Light at Night and Mistimed Food Intake Alter Hormonal Rhythms and Metabolism.Int J Mol Sci. 2023 Feb 8;24(4):3392. doi: 10.3390/ijms24043392. Int J Mol Sci. 2023. PMID: 36834801 Free PMC article. Review.
-
Regulation of stress-induced sleep fragmentation by preoptic glutamatergic neurons.Curr Biol. 2024 Jan 8;34(1):12-23.e5. doi: 10.1016/j.cub.2023.11.035. Epub 2023 Dec 13. Curr Biol. 2024. PMID: 38096820 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
