Ubiquitin-specific protease 53 promotes osteogenic differentiation of human bone marrow-derived mesenchymal stem cells
- PMID: 33664230
- PMCID: PMC7933275
- DOI: 10.1038/s41419-021-03517-x
Ubiquitin-specific protease 53 promotes osteogenic differentiation of human bone marrow-derived mesenchymal stem cells
Abstract
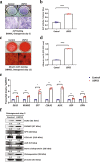
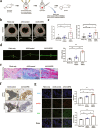
The ubiquitin protease pathway plays important role in human bone marrow-derived mesenchymal stem cell (hBMSC) differentiation, including osteogenesis. However, the function of deubiquitinating enzymes in osteogenic differentiation of hBMSCs remains poorly understood. In this study, we aimed to investigate the role of ubiquitin-specific protease 53 (USP53) in the osteogenic differentiation of hBMSCs. Based on re-analysis of the Gene Expression Omnibus database, USP53 was selected as a positive regulator of osteogenic differentiation in hBMSCs. Overexpression of USP53 by lentivirus enhanced osteogenesis in hBMSCs, whereas knockdown of USP53 by lentivirus inhibited osteogenesis in hBMSCs. In addition, USP53 overexpression increased the level of active β-catenin and enhanced the osteogenic differentiation of hBMSCs. This effect was reversed by the Wnt/β-catenin inhibitor DKK1. Mass spectrometry showed that USP53 interacted with F-box only protein 31 (FBXO31) to promote proteasomal degradation of β-catenin. Inhibition of the osteogenic differentiation of hBMSCs by FBXO31 was partially rescued by USP53 overexpression. Animal studies showed that hBMSCs with USP53 overexpression significantly promoted bone regeneration in mice with calvarial defects. These results suggested that USP53 may be a target for gene therapy for bone regeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
MFG-E8 promotes osteogenic differentiation of human bone marrow mesenchymal stem cells through GSK3β/β-catenin signaling pathway.FASEB J. 2023 Jun;37(6):e22950. doi: 10.1096/fj.202201417RRR. FASEB J. 2023. PMID: 37144883
-
Circ_FBLN1 promotes the proliferation and osteogenic differentiation of human bone marrow-derived mesenchymal stem cells by regulating let-7i-5p/FZD4 axis and Wnt/β-catenin pathway.J Bioenerg Biomembr. 2021 Oct;53(5):561-572. doi: 10.1007/s10863-021-09917-0. Epub 2021 Aug 23. J Bioenerg Biomembr. 2021. PMID: 34424449
-
MicroRNA-200c promotes osteogenic differentiation of human bone mesenchymal stem cells through activating the AKT/β-Catenin signaling pathway via downregulating Myd88.J Cell Physiol. 2019 Dec;234(12):22675-22686. doi: 10.1002/jcp.28834. Epub 2019 May 31. J Cell Physiol. 2019. PMID: 31152447
-
An Overview of the Deubiquitinase USP53: A Promising Diagnostic Marker and Therapeutic Target.Curr Protein Pept Sci. 2024;25(9):708-718. doi: 10.2174/0113892037292440240518194922. Curr Protein Pept Sci. 2024. PMID: 39300775 Free PMC article. Review.
-
Loss of hepatocyte Usp53 protects mice from a form of xenobiotic-induced liver injury.Biochim Biophys Acta Mol Basis Dis. 2025 Mar;1871(3):167624. doi: 10.1016/j.bbadis.2024.167624. Epub 2024 Dec 19. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 39705897 Review.
Cited by
-
RNF4~RGMb~BMP6 axis required for osteogenic differentiation and cancer cell survival.Cell Death Dis. 2022 Sep 24;13(9):820. doi: 10.1038/s41419-022-05262-1. Cell Death Dis. 2022. PMID: 36153321 Free PMC article.
-
Role of ubiquitination in the occurrence and development of osteoporosis (Review).Int J Mol Med. 2024 Aug;54(2):68. doi: 10.3892/ijmm.2024.5392. Epub 2024 Jun 28. Int J Mol Med. 2024. PMID: 38940355 Free PMC article. Review.
-
PERRC: Protease Engineering with Reactant Residence Time Control.bioRxiv [Preprint]. 2025 Mar 4:2025.03.02.641063. doi: 10.1101/2025.03.02.641063. bioRxiv. 2025. Update in: ACS Synth Biol. 2025 Jun 20;14(6):2241-2253. doi: 10.1021/acssynbio.5c00154. PMID: 40093119 Free PMC article. Updated. Preprint.
-
Regulatory Roles of E3 Ubiquitin Ligases and Deubiquitinases in Bone.Biomolecules. 2025 May 7;15(5):679. doi: 10.3390/biom15050679. Biomolecules. 2025. PMID: 40427572 Free PMC article. Review.
-
Strategies of Macrophages to Maintain Bone Homeostasis and Promote Bone Repair: A Narrative Review.J Funct Biomater. 2022 Dec 29;14(1):18. doi: 10.3390/jfb14010018. J Funct Biomater. 2022. PMID: 36662065 Free PMC article. Review.
References
-
- Bernard NJ. Sensing bone mass. Nat. Rev. Rheumatol. 2019;15:128. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

