Mesenchymal stem cells alleviate experimental immune-mediated liver injury via chitinase 3-like protein 1-mediated T cell suppression
- PMID: 33664231
- PMCID: PMC7933182
- DOI: 10.1038/s41419-021-03524-y
Mesenchymal stem cells alleviate experimental immune-mediated liver injury via chitinase 3-like protein 1-mediated T cell suppression
Abstract
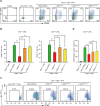
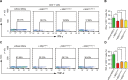
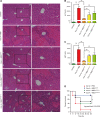
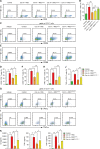
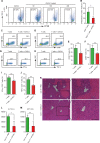
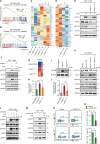
Liver diseases with different pathogenesis share common pathways of immune-mediated injury. Chitinase-3-like protein 1 (CHI3L1) was induced in both acute and chronic liver injuries, and recent studies reported that it possesses an immunosuppressive ability. CHI3L1 was also expressed in mesenchymal stem cells (MSCs), thus we investigates the role of CHI3L1 in MSC-based therapy for immune-mediated liver injury here. We found that CHI3L1 was highly expressed in human umbilical cord MSCs (hUC-MSCs). Downregulating CHI3L1 mitigated the ability of hUC-MSCs to inhibit T cell activation, proliferation and inflammatory cytokine secretion in vitro. Using Concanavalin A (Con A)-induced liver injury mouse model, we found that silencing CHI3L1 significantly abrogated the hUC-MSCs-mediated alleviation of liver injury, accompanying by weakened suppressive effects on infiltration and activation of hepatic T cells, and secretion of pro-inflammatory cytokines. In addition, recombinant CHI3L1 (rCHI3L1) administration inhibited the proliferation and function of activated T cells, and alleviated the Con A-induced liver injury in mice. Mechanistically, gene set enrichment analysis showed that JAK/STAT signalling pathway was one of the most significantly enriched gene pathways in T cells co-cultured with hUC-MSCs with CHI3L1 knockdown, and further study revealed that CHI3L1 secreted by hUC-MSCs inhibited the STAT1/3 signalling in T cells by upregulating peroxisome proliferator-activated receptor δ (PPARδ). Collectively, our data showed that CHI3L1 was a novel MSC-secreted immunosuppressive factor and provided new insights into therapeutic treatment of immune-mediated liver injury.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Human umbilical cord-derived mesenchymal stem cells improve the function of liver in rats with acute-on-chronic liver failure via downregulating Notch and Stat1/Stat3 signaling.Stem Cell Res Ther. 2021 Jul 13;12(1):396. doi: 10.1186/s13287-021-02468-6. Stem Cell Res Ther. 2021. PMID: 34256837 Free PMC article.
-
Crosstalk between mesenchymal stem cells and T regulatory cells is crucially important for the attenuation of acute liver injury.Liver Transpl. 2018 May;24(5):687-702. doi: 10.1002/lt.25049. Epub 2018 Apr 16. Liver Transpl. 2018. PMID: 29500914
-
Tonsil-derived mesenchymal stem cells alleviate concanavalin A-induced acute liver injury.Exp Cell Res. 2014 Aug 1;326(1):143-54. doi: 10.1016/j.yexcr.2014.06.007. Epub 2014 Jun 19. Exp Cell Res. 2014. PMID: 24954408
-
What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment?Stem Cell Res Ther. 2020 Dec 1;11(1):519. doi: 10.1186/s13287-020-02011-z. Stem Cell Res Ther. 2020. PMID: 33261658 Free PMC article. Review.
-
Inflammatory Effects and Regulatory Mechanisms of Chitinase-3-like-1 in Multiple Human Body Systems: A Comprehensive Review.Int J Mol Sci. 2024 Dec 15;25(24):13437. doi: 10.3390/ijms252413437. Int J Mol Sci. 2024. PMID: 39769202 Free PMC article. Review.
Cited by
-
Mesenchymal Stem Cells and Their Small Extracellular Vesicles as Crucial Immunological Efficacy for Hepatic Diseases.Front Immunol. 2022 May 6;13:880523. doi: 10.3389/fimmu.2022.880523. eCollection 2022. Front Immunol. 2022. PMID: 35603168 Free PMC article. Review.
-
Ferroptosis in acute liver Failure: Unraveling the hepcidin-ferroportin axis and therapeutic interventions.Redox Biol. 2025 Jul;84:103657. doi: 10.1016/j.redox.2025.103657. Epub 2025 May 8. Redox Biol. 2025. PMID: 40393152 Free PMC article.
-
Decellularized Porcine Aorta as a Scaffold for Human Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells in Tissue Engineering.Stem Cell Rev Rep. 2025 Jun;21(5):1540-1559. doi: 10.1007/s12015-025-10875-y. Epub 2025 Apr 14. Stem Cell Rev Rep. 2025. PMID: 40227487 Free PMC article.
-
Matrix stiffness-dependent regulation of immunomodulatory genes in human MSCs is associated with the lncRNA CYTOR.Proc Natl Acad Sci U S A. 2024 Aug 6;121(32):e2404146121. doi: 10.1073/pnas.2404146121. Epub 2024 Jul 29. Proc Natl Acad Sci U S A. 2024. PMID: 39074278 Free PMC article.
-
Engineering extracellular vesicles by three-dimensional dynamic culture of human mesenchymal stem cells.J Extracell Vesicles. 2022 Jun;11(6):e12235. doi: 10.1002/jev2.12235. J Extracell Vesicles. 2022. PMID: 35716062 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

