Impairment in inflammasome signaling by the chronic Pseudomonas aeruginosa isolates from cystic fibrosis patients results in an increase in inflammatory response
- PMID: 33664232
- PMCID: PMC7933143
- DOI: 10.1038/s41419-021-03526-w
Impairment in inflammasome signaling by the chronic Pseudomonas aeruginosa isolates from cystic fibrosis patients results in an increase in inflammatory response
Abstract
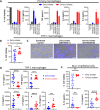
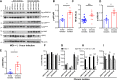
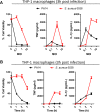
Pseudomonas aeruginosa is a common respiratory pathogen in cystic fibrosis (CF) patients which undergoes adaptations during chronic infection towards reduced virulence, which can facilitate bacterial evasion of killing by host cells. However, inflammatory cytokines are often found to be elevated in CF patients, and it is unknown how chronic P. aeruginosa infection can be paradoxically associated with both diminished virulence in vitro and increased inflammation and disease progression. Thus, we investigated the relationship between the stimulation of inflammatory cell death pathways by CF P. aeruginosa respiratory isolates and the expression of key inflammatory cytokines. We show that early respiratory isolates of P. aeruginosa from CF patients potently induce inflammasome signaling, cell death, and expression of IL-1β by macrophages, yet little expression of other inflammatory cytokines (TNF, IL-6 and IL-8). In contrast, chronic P. aeruginosa isolates induce relatively poor macrophage inflammasome signaling, cell death, and IL-1β expression but paradoxically excessive production of TNF, IL-6 and IL-8 compared to early P. aeruginosa isolates. Using various mutants of P. aeruginosa, we show that the premature cell death of macrophages caused by virulent bacteria compromises their ability to express cytokines. Contrary to the belief that chronic P. aeruginosa isolates are less pathogenic, we reveal that infections with chronic P. aeruginosa isolates result in increased cytokine induction due to their failure to induce immune cell death, which results in a relatively intense inflammation compared with early isolates.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

