E3 ubiquitin ligase RNF126 affects bladder cancer progression through regulation of PTEN stability
- PMID: 33664240
- PMCID: PMC7933351
- DOI: 10.1038/s41419-021-03521-1
E3 ubiquitin ligase RNF126 affects bladder cancer progression through regulation of PTEN stability
Abstract
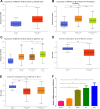
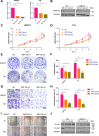
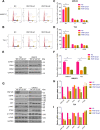
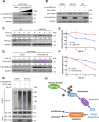
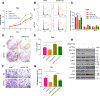
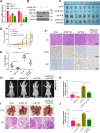
E3 ubiquitin ligase RNF126 (ring finger protein 126) is highly expressed in various cancers and strongly associated with tumorigenesis. However, its specific function in bladder cancer (BCa) is still debatable. Here, we found that RNF126 was significantly upregulated in BCa tissue by TCGA database, and our studies indicated that downregulation of RNF126 significantly inhibited cell proliferation and metastasis through the EGFR/PI3K/AKT signaling pathway in BCa cells. Furthermore, we identified PTEN, an inhibitor of the PI3K/AKT signaling pathway, as a novel substrate for RNF126. By co-immunoprecipitation assays, we proved that RNF126 directly interacts with PTEN. Predominantly, PTEN binds to the C-terminal containing the RING domain of RNF126. The in vivo ubiquitination assay showed that RNF126 specifically regulates PTEN stability through poly-ubiquitination. Furthermore, PTEN knockdown restored cell proliferation, metastasis, and tumor formation of BCa cells inhibited by RNF126 silencing in vitro and in vivo. In conclusion, these results identified RNF126 as an oncogene that functions through ubiquitination and degradation of PTEN in BCa.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Tumor cell-derived exosome RNF126 affects the immune microenvironment and promotes nasopharyngeal carcinoma progression by regulating PTEN ubiquitination.Apoptosis. 2022 Aug;27(7-8):590-605. doi: 10.1007/s10495-022-01738-9. Epub 2022 Jun 19. Apoptosis. 2022. PMID: 35717659
-
ZNF263 cooperates with ZNF31 to promote the drug resistance and EMT of pancreatic cancer through transactivating RNF126.J Cell Physiol. 2024 Jun;239(6):e31259. doi: 10.1002/jcp.31259. Epub 2024 Mar 22. J Cell Physiol. 2024. PMID: 38515383
-
IMP3 accelerates the progression of prostate cancer through inhibiting PTEN expression in a SMURF1-dependent way.J Exp Clin Cancer Res. 2020 Sep 16;39(1):190. doi: 10.1186/s13046-020-01657-0. J Exp Clin Cancer Res. 2020. Retraction in: J Exp Clin Cancer Res. 2023 Jan 17;42(1):24. doi: 10.1186/s13046-023-02599-z. PMID: 32938489 Free PMC article. Retracted.
-
Upregulation of Neural Precursor Cell Expressed Developmentally Downregulated 4-1 is Associated with Poor Prognosis and Chemoresistance in Lung Adenocarcinoma.Chin Med J (Engl). 2018 Jan 5;131(1):16-24. doi: 10.4103/0366-6999.221262. Chin Med J (Engl). 2018. PMID: 29271375 Free PMC article.
-
Roles of RNF126 and BCA2 E3 ubiquitin ligases in DNA damage repair signaling and targeted cancer therapy.Pharmacol Res. 2020 May;155:104748. doi: 10.1016/j.phrs.2020.104748. Epub 2020 Mar 6. Pharmacol Res. 2020. PMID: 32147403 Review.
Cited by
-
E3 ubiquitin ligases and deubiquitinases in bladder cancer tumorigenesis and implications for immunotherapies.Front Immunol. 2023 Jul 11;14:1226057. doi: 10.3389/fimmu.2023.1226057. eCollection 2023. Front Immunol. 2023. PMID: 37497216 Free PMC article. Review.
-
The role of E3 ubiquitin ligases and deubiquitinases in bladder cancer development and immunotherapy.Front Immunol. 2023 May 5;14:1202633. doi: 10.3389/fimmu.2023.1202633. eCollection 2023. Front Immunol. 2023. PMID: 37215134 Free PMC article. Review.
-
Ring finger protein 12 activates AKT signalling to promote the progression of liver cancer by interacting with EGFR.J Cell Mol Med. 2023 Jun;27(11):1523-1538. doi: 10.1111/jcmm.17757. Epub 2023 May 2. J Cell Mol Med. 2023. PMID: 37132043 Free PMC article.
-
Ubiquitin-proteasome system as a target for anticancer treatment-an update.Arch Pharm Res. 2023 Jul;46(7):573-597. doi: 10.1007/s12272-023-01455-0. Epub 2023 Aug 5. Arch Pharm Res. 2023. PMID: 37541992 Review.
-
Urinary exosomal lnc-TAF12-2:1 promotes bladder cancer progression through the miR-7847-3p/ASB12 regulatory axis.Genes Dis. 2024 Aug 5;12(4):101384. doi: 10.1016/j.gendis.2024.101384. eCollection 2025 Jul. Genes Dis. 2024. PMID: 40297540 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

