Epidermal growth factor receptor signaling uncouples germ cells from the somatic follicular compartment at ovulation
- PMID: 33664246
- PMCID: PMC7933413
- DOI: 10.1038/s41467-021-21644-z
Epidermal growth factor receptor signaling uncouples germ cells from the somatic follicular compartment at ovulation
Abstract
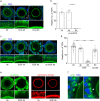
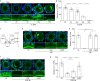
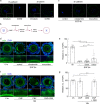
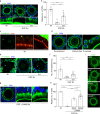
Germ cells are physically coupled to somatic support cells of the gonad during differentiation, but this coupling must be disrupted when they are mature, freeing them to participate in fertilization. In mammalian females, coupling occurs via specialized filopodia that project from the ovarian follicular granulosa cells to the oocyte. Here, we show that signaling through the epidermal growth factor receptor (EGFR) in the granulosa, which becomes activated at ovulation, uncouples the germ and somatic cells by triggering a massive and temporally synchronized retraction of the filopodia. Although EGFR signaling triggers meiotic maturation of the oocyte, filopodial retraction is independent of the germ cell state, being regulated solely within the somatic compartment, where it requires ERK-dependent calpain-mediated loss of filopodia-oocyte adhesion followed by Arp2/3-mediated filopodial shortening. By uncovering the mechanism regulating germ-soma uncoupling at ovulation, our results open a path to improving oocyte quality in human and animal reproduction.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles.Mol Reprod Dev. 2012 Nov;79(11):795-802. doi: 10.1002/mrd.22114. Epub 2012 Sep 28. Mol Reprod Dev. 2012. PMID: 22987720
-
The epidermal growth factor network: role in oocyte growth, maturation and developmental competence.Hum Reprod Update. 2018 Jan 1;24(1):1-14. doi: 10.1093/humupd/dmx029. Hum Reprod Update. 2018. PMID: 29029246 Review.
-
Ovulatory signals alter granulosa cell behavior through YAP1 signaling.Reprod Biol Endocrinol. 2019 Dec 28;17(1):113. doi: 10.1186/s12958-019-0552-1. Reprod Biol Endocrinol. 2019. PMID: 31883523 Free PMC article.
-
Significance of epidermal growth factor receptor signaling for acquisition of meiotic and developmental competence in mammalian oocytes.Biol Reprod. 2017 Oct 1;97(4):537-549. doi: 10.1093/biolre/iox112. Biol Reprod. 2017. PMID: 29025011 Review.
-
Mammalian Oocytes Locally Remodel Follicular Architecture to Provide the Foundation for Germline-Soma Communication.Curr Biol. 2018 Apr 2;28(7):1124-1131.e3. doi: 10.1016/j.cub.2018.02.039. Epub 2018 Mar 22. Curr Biol. 2018. PMID: 29576478 Free PMC article.
Cited by
-
Transcriptomic Profile of Early Antral Follicles: Predictive Somatic Gene Markers of Oocyte Maturation Outcome.Cells. 2025 May 12;14(10):704. doi: 10.3390/cells14100704. Cells. 2025. PMID: 40422207 Free PMC article.
-
Bidirectional communication in oogenesis: a dynamic conversation in mice and Drosophila.Trends Cell Biol. 2022 Apr;32(4):311-323. doi: 10.1016/j.tcb.2021.11.005. Epub 2021 Dec 16. Trends Cell Biol. 2022. PMID: 34922803 Free PMC article. Review.
-
N-cadherin mechanosensing in ovarian follicles controls oocyte maturation and ovulation.Elife. 2025 Jul 29;13:RP92068. doi: 10.7554/eLife.92068. Elife. 2025. PMID: 40728874 Free PMC article.
-
Regulation of oocyte maturation: Role of conserved ERK signaling.Mol Reprod Dev. 2022 Sep;89(9):353-374. doi: 10.1002/mrd.23637. Epub 2022 Jul 31. Mol Reprod Dev. 2022. PMID: 35908193 Free PMC article. Review.
-
Regulating human oocyte maturation in vitro: a hypothesis based on oocytes retrieved from small antral follicles during ovarian tissue cryopreservation.J Assist Reprod Genet. 2025 May;42(5):1461-1472. doi: 10.1007/s10815-025-03483-9. Epub 2025 Apr 22. J Assist Reprod Genet. 2025. PMID: 40261459 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

