Virus-specific memory T cell responses unmasked by immune checkpoint blockade cause hepatitis
- PMID: 33664251
- PMCID: PMC7933278
- DOI: 10.1038/s41467-021-21572-y
Virus-specific memory T cell responses unmasked by immune checkpoint blockade cause hepatitis
Abstract
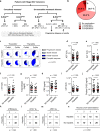
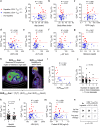
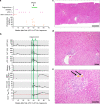
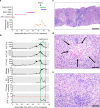
Treatment of advanced melanoma with combined PD-1/CTLA-4 blockade commonly causes serious immune-mediated complications. Here, we identify a subset of patients predisposed to immune checkpoint blockade-related hepatitis who are distinguished by chronic expansion of effector memory CD4+ T cells (TEM cells). Pre-therapy CD4+ TEM cell expansion occurs primarily during autumn or winter in patients with metastatic disease and high cytomegalovirus (CMV)-specific serum antibody titres. These clinical features implicate metastasis-dependent, compartmentalised CMV reactivation as the cause of CD4+ TEM expansion. Pre-therapy CD4+ TEM expansion predicts hepatitis in CMV-seropositive patients, opening possibilities for avoidance or prevention. 3 of 4 patients with pre-treatment CD4+ TEM expansion who received αPD-1 monotherapy instead of αPD-1/αCTLA-4 therapy remained hepatitis-free. 4 of 4 patients with baseline CD4+ TEM expansion given prophylactic valganciclovir and αPD-1/αCTLA-4 therapy remained hepatitis-free. Our findings exemplify how pathogen exposure can shape clinical reactions after cancer therapy and how this insight leads to therapeutic innovations.
Conflict of interest statement
S.H. declares he has received consulting fees and speaker’s honoraria from BMS and Merck Sharp & Dohme (MSD). All other authors declare no competing interests.
Figures





Comment in
-
Could controlling occult cytomegalovirus reactivation with prophylactic valganciclovir prevent immune checkpoint blockade-Related complications?Eur J Cancer. 2021 Aug;153:72-73. doi: 10.1016/j.ejca.2021.05.009. Epub 2021 Jun 18. Eur J Cancer. 2021. PMID: 34153716 No abstract available.
Similar articles
-
Prediction of immune checkpoint blockade-related hepatitis in metastatic melanoma patients.J Dtsch Dermatol Ges. 2022 Jun;20(6):773-775. doi: 10.1111/ddg.14726. Epub 2022 May 2. J Dtsch Dermatol Ges. 2022. PMID: 35499199 Review.
-
Development of a Flow Cytometry Assay to Predict Immune Checkpoint Blockade-Related Complications.Front Immunol. 2021 Nov 16;12:765644. doi: 10.3389/fimmu.2021.765644. eCollection 2021. Front Immunol. 2021. PMID: 34868015 Free PMC article.
-
Differential effects of PD-1 and CTLA-4 blockade on the melanoma-reactive CD8 T cell response.Proc Natl Acad Sci U S A. 2021 Oct 26;118(43):e2102849118. doi: 10.1073/pnas.2102849118. Proc Natl Acad Sci U S A. 2021. PMID: 34670835 Free PMC article.
-
Could a loss of memory T cells limit responses to hepatitis C virus (HCV) antigens in blood leucocytes from patients chronically infected with HCV before and during pegylated interferon-alpha and ribavirin therapy?Clin Exp Immunol. 2010 Jul 1;161(1):118-26. doi: 10.1111/j.1365-2249.2010.04141.x. Epub 2010 Apr 9. Clin Exp Immunol. 2010. PMID: 20408862 Free PMC article.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
Cited by
-
Platelet apheresis with additive solution and plasma rinseback affects the cellular composition of LRS chamber products.Sci Rep. 2025 Jun 6;15(1):19923. doi: 10.1038/s41598-025-04350-4. Sci Rep. 2025. PMID: 40481081 Free PMC article.
-
Extracorporeal photopheresis for pembrolizumab-induced dermatitis: a case report.Skin Health Dis. 2025 Jun 25;5(4):293-296. doi: 10.1093/skinhd/vzaf045. eCollection 2025 Aug. Skin Health Dis. 2025. PMID: 40755883 Free PMC article.
-
Toxicity-specific peripheral blood T and B cell dynamics in anti-PD-1 and combined immune checkpoint inhibition.Cancer Immunol Immunother. 2023 Dec;72(12):4049-4064. doi: 10.1007/s00262-023-03541-0. Epub 2023 Oct 4. Cancer Immunol Immunother. 2023. PMID: 37794264 Free PMC article.
-
What do cancer-specific T cells 'see'?Discov Immunol. 2022 Dec 6;2(1):kyac011. doi: 10.1093/discim/kyac011. eCollection 2023. Discov Immunol. 2022. PMID: 38567060 Free PMC article. Review.
-
Immune-mediated liver injury from checkpoint inhibitors: mechanisms, clinical characteristics and management.Nat Rev Gastroenterol Hepatol. 2025 Feb;22(2):112-126. doi: 10.1038/s41575-024-01019-7. Epub 2024 Dec 11. Nat Rev Gastroenterol Hepatol. 2025. PMID: 39663461 Review.
References
-
- Larkin, J. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med.381, 1535–1546 (2019). - PubMed
-
- Robert C, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239–1251. - PubMed
-
- Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372:320–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

