Long noncoding RNA LINC00518 induces radioresistance by regulating glycolysis through an miR-33a-3p/HIF-1α negative feedback loop in melanoma
- PMID: 33664256
- PMCID: PMC7933330
- DOI: 10.1038/s41419-021-03523-z
Long noncoding RNA LINC00518 induces radioresistance by regulating glycolysis through an miR-33a-3p/HIF-1α negative feedback loop in melanoma
Abstract
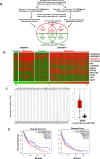
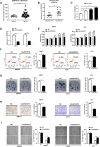
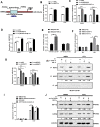
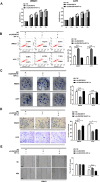
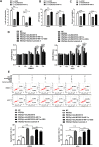
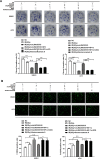
The long noncoding RNA, LINC00518, is highly expressed in various types of cancers and is involved in cancer progression. Although LINC00518 promotes the metastasis of cutaneous malignant melanoma (CMM), the mechanism underlaying its effects on CMM radiosensitivity remains unclear. In this study, LINC00518 expression was significantly upregulated in CMM samples, and LINC00518 levels were associated with poor prognosis of patients with CMM. Knockdown of LINC00518 in CMM cells significantly inhibited cell invasion, migration, proliferation, and clonogenicity. LINC00518-mediated invasion, migration, proliferation, and clonogenicity were negatively regulated by the microRNA, miR-33a-3p, in vitro, which increased sensitivity to radiotherapy via inhibition of the hypoxia-inducible factor 1α (HIF-1α)/lactate dehydrogenase A glycolysis axis. Additionally, HIF-1α recognized the miR-33a-3p promoter region and recruited histone deacetylase 2, which decreased the expression of miR-33a-3p and formed an LINC00518/miR-33a-3p/HIF-1α negative feedback loop. Furthermore, signaling with initially activated glycolysis and radioresistance in CMM cells was impaired by Santacruzamate A, a histone deacetylase inhibitor, and 2-deoxy-D-glucose, a glycolytic inhibitor. Lastly, knockdown of LINC00518 expression sensitized CMM cancer cells to radiotherapy in an in vivo subcutaneously implanted tumor model. In conclusion, LINC00518 was confirmed to be an oncogene in CMM, which induces radioresistance by regulating glycolysis through an miR-33a-3p/HIF-1α negative feedback loop. Our study, may provide a potential strategy to improve the treatment outcome of radiotherapy in CMM.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
LINC00518 affects the proliferation, invasion and migration of cutaneous malignant melanoma cells via miR-526b-3p/EIF5A2 axis.Acta Biochim Pol. 2022 Feb 21;69(1):101-111. doi: 10.18388/abp.2020_5746. Acta Biochim Pol. 2022. PMID: 35189053
-
Dysregulation of LncRNA ANRIL mediated by miR-411-3p inhibits the malignant proliferation and tumor stem cell like property of multiple myeloma via hypoxia-inducible factor 1α.Exp Cell Res. 2020 Nov 1;396(1):112280. doi: 10.1016/j.yexcr.2020.112280. Epub 2020 Sep 19. Exp Cell Res. 2020. PMID: 32961145
-
miR-33a functions as a tumor suppressor in melanoma by targeting HIF-1α.Cancer Biol Ther. 2015;16(6):846-55. doi: 10.1080/15384047.2015.1030545. Cancer Biol Ther. 2015. PMID: 25891797 Free PMC article.
-
Unraveling the role of hypoxia-inducible factors in cutaneous melanoma: from mechanisms to therapeutic opportunities.Cell Commun Signal. 2025 Apr 9;23(1):177. doi: 10.1186/s12964-025-02173-4. Cell Commun Signal. 2025. PMID: 40205422 Free PMC article. Review.
-
Interactions Between Non-Coding RNAs and HIF-1alpha in the Context of Colorectal Cancer.Biomolecules. 2025 Apr 1;15(4):510. doi: 10.3390/biom15040510. Biomolecules. 2025. PMID: 40305214 Free PMC article. Review.
Cited by
-
Development and validation of a ferroptosis-related lncRNAs signature to predict prognosis and microenvironment for melanoma.Discov Oncol. 2022 Nov 12;13(1):125. doi: 10.1007/s12672-022-00581-3. Discov Oncol. 2022. PMID: 36371574 Free PMC article.
-
Long noncoding RNAs, glucose metabolism and cancer (Review).Oncol Lett. 2023 Jun 22;26(2):340. doi: 10.3892/ol.2023.13925. eCollection 2023 Aug. Oncol Lett. 2023. PMID: 37427347 Free PMC article. Review.
-
Regulatory Mechanisms of LncRNAs in Cancer Glycolysis: Facts and Perspectives.Cancer Manag Res. 2021 Jul 5;13:5317-5336. doi: 10.2147/CMAR.S314502. eCollection 2021. Cancer Manag Res. 2021. PMID: 34262341 Free PMC article. Review.
-
Santacruzamate A Alleviates Pain and Pain-Related Adverse Emotions through the Inhibition of Microglial Activation in the Anterior Cingulate Cortex.ACS Pharmacol Transl Sci. 2024 Feb 16;7(4):1002-1012. doi: 10.1021/acsptsci.3c00282. eCollection 2024 Apr 12. ACS Pharmacol Transl Sci. 2024. PMID: 38633586 Free PMC article.
-
IGF2BP2 regulates gastric cancer radiotherapy resistance through HIF1α-mediated glycolysis.Front Oncol. 2025 May 16;15:1512177. doi: 10.3389/fonc.2025.1512177. eCollection 2025. Front Oncol. 2025. PMID: 40469197 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

