Distinct effects of V617F and exon12-mutated JAK2 expressions on erythropoiesis in a human induced pluripotent stem cell (iPSC)-based model
- PMID: 33664283
- PMCID: PMC7933160
- DOI: 10.1038/s41598-021-83895-6
Distinct effects of V617F and exon12-mutated JAK2 expressions on erythropoiesis in a human induced pluripotent stem cell (iPSC)-based model
Abstract
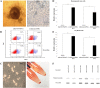
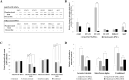
Activating mutations affecting the JAK-STAT signal transduction is the genetic driver of myeloproliferative neoplasms (MPNs) which comprise polycythemia vera (PV), essential thrombocythemia (ET) and myelofibrosis. The JAK2p.V617F mutation can produce both erythrocytosis in PV and thrombocytosis in ET, while JAK2 exon 12 mutations cause only erythrocytosis. We hypothesized that these two mutations activated different intracellular signals. In this study, the induced pluripotent stem cells (iPSCs) were used to model JAK2-mutated MPNs. Normal iPSCs underwent lentiviral transduction to overexpress JAK2p.V617F or JAK2p.N542_E543del (JAK2exon12) under a doxycycline-inducible system. The modified iPSCs were differentiated into erythroid cells. Compared with JAK2V617F-iPSCs, JAK2exon12-iPSCs yielded more total CD71+GlycophorinA+ erythroid cells, displayed more mature morphology and expressed more adult hemoglobin after doxycycline induction. Capillary Western immunoassay revealed significantly higher phospho-STAT1 but lower phospho-STAT3 and lower Phospho-AKT in JAK2exon12-iPSCs compared with those of JAK2V617F-iPSCs in response to erythropoietin. Furthermore, interferon alpha and arsenic trioxide were tested on these modified iPSCs to explore their potentials for MPN therapy. Both agents preferentially inhibited proliferation and promoted apoptosis of the iPSCs expressing mutant JAK2 compared with those without doxycycline induction. In conclusion, the modified iPSC model can be used to investigate the mechanisms and search for new therapy of MPNs.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Mutated JAK2 signal transduction in human induced pluripotent stem cell (iPSC)-derived megakaryocytes.Platelets. 2022 Jul 4;33(5):700-708. doi: 10.1080/09537104.2021.1981850. Epub 2021 Nov 9. Platelets. 2022. PMID: 34749590
-
[Myeloproliferative diseases caused by JAK2 mutation].Rinsho Byori. 2009 Apr;57(4):357-64. Rinsho Byori. 2009. PMID: 19489438 Review. Japanese.
-
Loss of Stat1 decreases megakaryopoiesis and favors erythropoiesis in a JAK2-V617F-driven mouse model of MPNs.Blood. 2014 Jun 19;123(25):3943-50. doi: 10.1182/blood-2013-07-514208. Epub 2014 May 12. Blood. 2014. PMID: 24820309
-
JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis.N Engl J Med. 2007 Feb 1;356(5):459-68. doi: 10.1056/NEJMoa065202. N Engl J Med. 2007. PMID: 17267906 Free PMC article.
-
Changing concepts of diagnostic criteria of myeloproliferative disorders and the molecular etiology and classification of myeloproliferative neoplasms: from Dameshek 1950 to Vainchenker 2005 and beyond.Acta Haematol. 2015;133(1):36-51. doi: 10.1159/000358580. Epub 2014 Aug 7. Acta Haematol. 2015. PMID: 25116092 Review.
Cited by
-
CRISPR/Cas9-Based Modeling of JAK2 V617F Mutation in K562 Cells Reveals Enhanced Proliferation and Sensitivity to Therapeutic Agents.Int J Mol Sci. 2025 May 11;26(10):4600. doi: 10.3390/ijms26104600. Int J Mol Sci. 2025. PMID: 40429745 Free PMC article.
-
Advances in Molecular Understanding of Polycythemia Vera, Essential Thrombocythemia, and Primary Myelofibrosis: Towards Precision Medicine.Cancers (Basel). 2024 Apr 26;16(9):1679. doi: 10.3390/cancers16091679. Cancers (Basel). 2024. PMID: 38730632 Free PMC article. Review.
-
Current Advances in the Diagnosis and Treatment of Major Myeloproliferative Neoplasms.Cancers (Basel). 2025 May 30;17(11):1834. doi: 10.3390/cancers17111834. Cancers (Basel). 2025. PMID: 40507313 Free PMC article. Review.
-
Harnessing the Power of Induced Pluripotent Stem Cells and Gene Editing Technology: Therapeutic Implications in Hematological Malignancies.Cells. 2021 Oct 9;10(10):2698. doi: 10.3390/cells10102698. Cells. 2021. PMID: 34685678 Free PMC article. Review.
-
Recent Progress in Interferon Therapy for Myeloid Malignancies.Front Oncol. 2021 Oct 29;11:769628. doi: 10.3389/fonc.2021.769628. eCollection 2021. Front Oncol. 2021. PMID: 34778087 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

