Knocking out alpha-synuclein in melanoma cells dysregulates cellular iron metabolism and suppresses tumor growth
- PMID: 33664298
- PMCID: PMC7933179
- DOI: 10.1038/s41598-021-84443-y
Knocking out alpha-synuclein in melanoma cells dysregulates cellular iron metabolism and suppresses tumor growth
Abstract
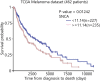
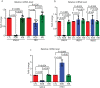
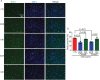
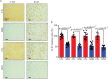
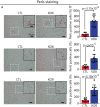
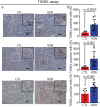
The protein alpha-synuclein (α-syn) is unusual because, depending on its conformation and the type of cell in which it is expressed, it is pro-death or pro-survival, triggering neurodegeneration in Parkinson's disease and enhancing cell survival of some melanomas. To probe the function of α-syn in melanoma, we used CRISPR/Cas9 to knockout SNCA, the gene that codes for α-syn, in SK-Mel-28 melanoma cells. The SNCA-knockout clones in culture exhibited a decrease in the transferrin receptor 1 (TfR1), an increase in ferritin, an increase of reactive oxygen species and proliferated slower than control cells. These SNCA-knockout clones grafted into SCID mice grew significantly slower than the SK-Mel-28 control cells that expressed α-syn. In the excised SNCA-knockout xenografts, TfR1 decreased 3.3-fold, ferritin increased 6.2-fold, the divalent metal ion transporter 1 (DMT1) increased threefold, and the iron exporter ferroportin (FPN1) decreased twofold relative to control xenografts. The excised SNCA-KO tumors exhibited significantly more ferric iron and TUNEL staining relative to the control melanoma xenografts. Collectively, depletion of α-syn in SK-Mel-28 cells dysregulates cellular iron metabolism, especially in xenografts, yielding melanoma cells that are deficient in TfR1 and FPN1, that accumulate ferric iron and ferritin, and that undergo apoptosis relative to control cells expressing α-syn.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Knocking out alpha-synuclein in melanoma cells downregulates L1CAM and decreases motility.Sci Rep. 2023 Jun 7;13(1):9243. doi: 10.1038/s41598-023-36451-3. Sci Rep. 2023. PMID: 37286800 Free PMC article.
-
Alpha-synuclein modulates the positioning of endolysosomes in melanoma cells.Hum Mol Genet. 2025 Aug 21;34(17):1433-1445. doi: 10.1093/hmg/ddaf096. Hum Mol Genet. 2025. PMID: 40521809 Free PMC article.
-
Iron Metabolism in the Peripheral Nervous System: The Role of DMT1, Ferritin, and Transferrin Receptor in Schwann Cell Maturation and Myelination.J Neurosci. 2019 Dec 11;39(50):9940-9953. doi: 10.1523/JNEUROSCI.1409-19.2019. Epub 2019 Nov 1. J Neurosci. 2019. PMID: 31676601 Free PMC article.
-
Cellular Iron Metabolism and Regulation.Adv Exp Med Biol. 2019;1173:21-32. doi: 10.1007/978-981-13-9589-5_2. Adv Exp Med Biol. 2019. PMID: 31456203 Review.
-
Molecular regulation of cellular iron balance.IUBMB Life. 2017 Jun;69(6):389-398. doi: 10.1002/iub.1628. Epub 2017 May 7. IUBMB Life. 2017. PMID: 28480557 Review.
Cited by
-
Exploring α-Syn's Functions Through Ablation Models: Physiological and Pathological Implications.Cell Mol Neurobiol. 2025 May 19;45(1):44. doi: 10.1007/s10571-025-01560-2. Cell Mol Neurobiol. 2025. PMID: 40389720 Free PMC article. Review.
-
Linking Parkinson's Disease and Melanoma: Interplay Between α-Synuclein and Pmel17 Amyloid Formation.Mov Disord. 2021 Jul;36(7):1489-1498. doi: 10.1002/mds.28655. Epub 2021 May 22. Mov Disord. 2021. PMID: 34021920 Free PMC article. Review.
-
Occurrence of Total and Proteinase K-Resistant Alpha-Synuclein in Glioblastoma Cells Depends on mTOR Activity.Cancers (Basel). 2022 Mar 8;14(6):1382. doi: 10.3390/cancers14061382. Cancers (Basel). 2022. PMID: 35326535 Free PMC article.
-
Iron homeostasis imbalance and ferroptosis in brain diseases.MedComm (2020). 2023 Jun 26;4(4):e298. doi: 10.1002/mco2.298. eCollection 2023 Aug. MedComm (2020). 2023. PMID: 37377861 Free PMC article. Review.
-
Knocking out alpha-synuclein in melanoma cells downregulates L1CAM and decreases motility.Sci Rep. 2023 Jun 7;13(1):9243. doi: 10.1038/s41598-023-36451-3. Sci Rep. 2023. PMID: 37286800 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

