Epithelial to mesenchymal transition and microRNA expression are associated with spindle and apocrine cell morphology in triple-negative breast cancer
- PMID: 33664322
- PMCID: PMC7933252
- DOI: 10.1038/s41598-021-84350-2
Epithelial to mesenchymal transition and microRNA expression are associated with spindle and apocrine cell morphology in triple-negative breast cancer
Abstract
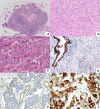
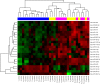
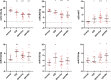
Triple negative breast cancers (TNBC) are a morphologically and genetically heterogeneous group of breast cancers with uncertain prediction of biological behavior and response to therapy. Epithelial to mesenchymal transition (EMT) is a dynamic process characterized by loss of typical epithelial phenotype and acquisition of mesenchymal characteristics. Aberrant activation of EMT can aggravate the prognosis of patients with cancer, however, the mechanisms of EMT and role of microRNAs (miRNAs) in EMT activation is still unclear. The aim of our study was to analyze miRNA expression within areas of TNBCs with cellular morphology that may be related to the EMT process and discuss possible associations. Out of all 3953 re-examined breast cancers, 460 breast cancers were diagnosed as TNBC (11.64%). With regard to complete tumor morphology preservation, the tissue samples obtained from core-cut biopsies and influenced by previous neoadjuvant therapy were excluded. We assembled a set of selected 25 cases to determine miRNA expression levels in relation to present focal spindle cell and apocrine cell morphology within individual TNBCs. We used descriptive (histological typing and morphology), morphometric, molecular (microdissection of tumor and non-tumor morphologies, RNA isolation and purification, microchip analysis) and bioinformatic analysis (including pathway analysis). The results were verified by quantitative real-time PCR (RT-qPCR) on an extended set of 70 TNBCs. The majority of TNBCs were represented by high-grade invasive carcinomas of no special type (NST) with medullary features characterized by well-circumscribed tumors with central necrosis or fibrosis and frequent tendency to spindle-cell and/or apocrine cell transformation. Apocrine and spindle cell transformation showed a specific miRNA expression profile in comparison to other tumor parts, in situ carcinoma or non-tumor structures, particularly down-regulated expression of hsa-miRNA-143-3p and hsa-miRNA-205-5p and up-regulated expression of hsa-miR-22-3p, hsa-miRNA-185-5p, and hsa-miR-4443. Apocrine cell tumor morphology further revealed decreased expression of hsa-miR-145-5p and increased expression of additional 14 miRNAs (e.g. hsa-miR-182-5p, hsa-miR-3135b and hsa-miR-4417). Pathway analysis for target genes of these miRNAs revealed several shared biological processes (i.e. Wnt signaling, ErbB signaling, MAPK signaling, endocytosis and axon guidance), which may in part contribute to the EMT and tumor progression. We provide the first miRNA expression profiling of specific tissue morphologies in TNBC. Our results demonstrate a specific miRNA expression profile of apocrine and spindle cell morphology which can exhibit a certain similarity with the EMT process and may also be relevant for prognosis and therapy resistance of TNBC.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

