The food additive EDTA aggravates colitis and colon carcinogenesis in mouse models
- PMID: 33664327
- PMCID: PMC7933154
- DOI: 10.1038/s41598-021-84571-5
The food additive EDTA aggravates colitis and colon carcinogenesis in mouse models
Abstract
Inflammatory bowel disease is a group of conditions with rising incidence caused by genetic and environmental factors including diet. The chelator ethylenediaminetetraacetate (EDTA) is widely used by the food and pharmaceutical industry among numerous other applications, leading to a considerable environmental exposure. Numerous safety studies in healthy animals have revealed no relevant toxicity by EDTA. Here we show that, in the presence of intestinal inflammation, EDTA is surprisingly capable of massively exacerbating inflammation and even inducing colorectal carcinogenesis at doses that are presumed to be safe. This toxicity is evident in two biologically different mouse models of inflammatory bowel disease, the AOM/DSS and the IL10-/- model. The mechanism of this effect may be attributed to disruption of intercellular contacts as demonstrated by in vivo confocal endomicroscopy, electron microscopy and cell culture studies. Our findings add EDTA to the list of food additives that might be detrimental in the presence of intestinal inflammation, but the toxicity of which may have been missed by regulatory safety testing procedures that utilize only healthy models. We conclude that the current use of EDTA especially in food and pharmaceuticals should be reconsidered. Moreover, we suggest that intestinal inflammatory models should be implemented in the testing of food additives to account for the exposure of this primary organ to environmental and dietary stress.
Conflict of interest statement
Rayko Evstatiev has received speaker’s honoraries from AoP Orphan and Vifor International. Rayko Evstatiev has received consultancy honoraries from AoP Orphan. Christoph Gasche has received research funding from AoP Orphan and Biogena within the Christian Doppler Laboratory on Molecular Cancer Chemoprevention. The other authors have no competing interests.
Figures

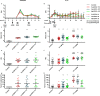
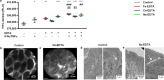

References
-
- European Union Risk Assessment Report edetic acid (EDTA). European Chemicals Agency Vol. 49 (2004).
-
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific opinion on the use of ferric sodium EDTA as a source of iron added for nutritional purposes to foods for the general population (including food supplements) and to foods for particular nutritional uses. EFSA J.8, 1414, 10.2903/j.efsa.2010.1414 (2010).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

