Bank vole prion protein extends the use of RT-QuIC assays to detect prions in a range of inherited prion diseases
- PMID: 33664355
- PMCID: PMC7933407
- DOI: 10.1038/s41598-021-84527-9
Bank vole prion protein extends the use of RT-QuIC assays to detect prions in a range of inherited prion diseases
Abstract
The cerebrospinal fluid (CSF) real-time quaking-induced conversion assay (RT-QuIC) is an ultrasensitive prion amyloid seeding assay for diagnosis of sporadic Creutzfeldt-Jakob disease (CJD) but several prion strains remain unexplored or resistant to conversion with commonly used recombinant prion protein (rPrP) substrates. Here, bank vole (BV) rPrP was used to study seeding by a wide range of archived post-mortem human CSF samples from cases of sporadic, acquired and various inherited prion diseases in high throughput 384-well format. BV rPrP substrate yielded positive reactions in 70/79 cases of sporadic CJD [Sensitivity 88.6% (95% CI 79.5-94.7%)], 1/2 variant CJD samples, and 9/20 samples from various inherited prion diseases; 5/57 non-prion disease control CSFs had positive reactions, yielding an overall specificity of 91.2% (95% CI 80.1-97.1%). Despite limitations of using post-mortem samples and our results' discrepancy with other studies, we demonstrated for the first time that BV rPrP is susceptible to conversion by human CSF samples containing certain prion strains not previously responsive in conventional rPrPs, thus justifying further optimisation for wider diagnostic and prognostic use.
Conflict of interest statement
The authors declare no competing interests.
Figures
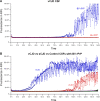
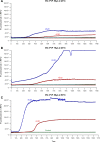

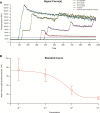
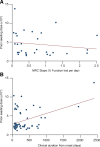
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

