Entropy-driven binding of gut bacterial β-glucuronidase inhibitors ameliorates irinotecan-induced toxicity
- PMID: 33664385
- PMCID: PMC7933434
- DOI: 10.1038/s42003-021-01815-w
Entropy-driven binding of gut bacterial β-glucuronidase inhibitors ameliorates irinotecan-induced toxicity
Abstract
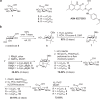
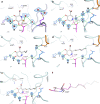
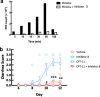
Irinotecan inhibits cell proliferation and thus is used for the primary treatment of colorectal cancer. Metabolism of irinotecan involves incorporation of β-glucuronic acid to facilitate excretion. During transit of the glucuronidated product through the gastrointestinal tract, an induced upregulation of gut microbial β-glucuronidase (GUS) activity may cause severe diarrhea and thus force many patients to stop treatment. We herein report the development of uronic isofagomine (UIFG) derivatives that act as general, potent inhibitors of bacterial GUSs, especially those of Escherichia coli and Clostridium perfringens. The best inhibitor, C6-nonyl UIFG, is 23,300-fold more selective for E. coli GUS than for human GUS (Ki = 0.0045 and 105 μM, respectively). Structural evidence indicated that the loss of coordinated water molecules, with the consequent increase in entropy, contributes to the high affinity and selectivity for bacterial GUSs. The inhibitors also effectively reduced irinotecan-induced diarrhea in mice without damaging intestinal epithelial cells.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Targeted inhibition of gut bacterial β-glucuronidase activity enhances anticancer drug efficacy.Proc Natl Acad Sci U S A. 2020 Mar 31;117(13):7374-7381. doi: 10.1073/pnas.1918095117. Epub 2020 Mar 13. Proc Natl Acad Sci U S A. 2020. PMID: 32170007 Free PMC article.
-
Substituent Position of Iminocyclitols Determines the Potency and Selectivity for Gut Microbial Xenobiotic-Reactivating Enzymes.J Med Chem. 2020 May 14;63(9):4617-4627. doi: 10.1021/acs.jmedchem.9b01918. Epub 2020 Mar 9. J Med Chem. 2020. PMID: 32105467
-
Irinotecan-gut microbiota interactions and the capability of probiotics to mitigate Irinotecan-associated toxicity.BMC Microbiol. 2023 Mar 2;23(1):53. doi: 10.1186/s12866-023-02791-3. BMC Microbiol. 2023. PMID: 36864380 Free PMC article.
-
Intestinal bacterial β-glucuronidase as a possible predictive biomarker of irinotecan-induced diarrhea severity.Pharmacol Ther. 2019 Jul;199:1-15. doi: 10.1016/j.pharmthera.2019.03.002. Epub 2019 Mar 1. Pharmacol Ther. 2019. PMID: 30831128 Review.
-
The role of gut microbial β-glucuronidases in carcinogenesis and cancer treatment: a scoping review.J Cancer Res Clin Oncol. 2024 Nov 13;150(11):495. doi: 10.1007/s00432-024-06028-2. J Cancer Res Clin Oncol. 2024. PMID: 39537966 Free PMC article.
Cited by
-
Inhibitory Activity of Bioactive Phloroglucinols from the Rhizomes of Dryopteris crassirhizoma on Escherichia coli β-Glucuronidase: Kinetic Analysis and Molecular Docking Studies.Metabolites. 2022 Oct 2;12(10):938. doi: 10.3390/metabo12100938. Metabolites. 2022. PMID: 36295840 Free PMC article.
-
Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes.Gut. 2022 Jul;71(7):1412-1425. doi: 10.1136/gutjnl-2021-326264. Epub 2022 Mar 11. Gut. 2022. PMID: 35277453 Free PMC article. Review.
-
Targeted inhibition of Gus-expressing Enterococcus faecalis to promote intestinal stem cell and epithelial renovation contributes to the relief of irinotecan chemotoxicity by dehydrodiisoeugenol.Acta Pharm Sin B. 2024 Dec;14(12):5286-5304. doi: 10.1016/j.apsb.2024.09.018. Epub 2024 Oct 18. Acta Pharm Sin B. 2024. PMID: 39807321 Free PMC article.
-
Gut microbial β-glucuronidases influence endobiotic homeostasis and are modulated by diverse therapeutics.Cell Host Microbe. 2024 Jun 12;32(6):925-944.e10. doi: 10.1016/j.chom.2024.04.018. Epub 2024 May 15. Cell Host Microbe. 2024. PMID: 38754417 Free PMC article.
-
Microbiota in cancer: molecular mechanisms and therapeutic interventions.MedComm (2020). 2023 Nov 5;4(6):e417. doi: 10.1002/mco2.417. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 37937304 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

