Smart wearable devices in cardiovascular care: where we are and how to move forward
- PMID: 33664502
- PMCID: PMC7931503
- DOI: 10.1038/s41569-021-00522-7
Smart wearable devices in cardiovascular care: where we are and how to move forward
Abstract
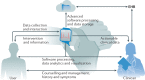
Technological innovations reach deeply into our daily lives and an emerging trend supports the use of commercial smart wearable devices to manage health. In the era of remote, decentralized and increasingly personalized patient care, catalysed by the COVID-19 pandemic, the cardiovascular community must familiarize itself with the wearable technologies on the market and their wide range of clinical applications. In this Review, we highlight the basic engineering principles of common wearable sensors and where they can be error-prone. We also examine the role of these devices in the remote screening and diagnosis of common cardiovascular diseases, such as arrhythmias, and in the management of patients with established cardiovascular conditions, for example, heart failure. To date, challenges such as device accuracy, clinical validity, a lack of standardized regulatory policies and concerns for patient privacy are still hindering the widespread adoption of smart wearable technologies in clinical practice. We present several recommendations to navigate these challenges and propose a simple and practical 'ABCD' guide for clinicians, personalized to their specific practice needs, to accelerate the integration of these devices into the clinical workflow for optimal patient care.
© 2021. Springer Nature Limited.
Conflict of interest statement
F.A.M. and S.S.M. are co-founders of and hold equity in Corrie Health. Under a licence agreement between Corrie Health and the Johns Hopkins University, the University owns equity in Corrie Health, and the University, F.A.M. and S.S.M. are entitled to royalty distributions related to technology described in the study discussed in this publication. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. S.S.M. has received consulting and advisory fees from 89bio, Akcea, Amgen, Astra Zeneca, Esperion, Kaneka, Novo Nordisk, Quest Diagnostics, Sanofi, Regeneron and REGENXBIO and funding from Amgen. M.P.T. has received funding from Apple, AstraZeneca and Boehringer Ingelheim, consulting fees from Abbott, Biotronik, Cardiva Medical, Johnson and Johnson, Medtronic and Novartis, and is an editor for
Figures

References
-
- Polaris Market Research. Healthcare analytics market share, size, trends, industry analysis report, 2021–2028. Polaris Market Researchhttps://www.polarismarketresearch.com/industry-analysis/wearable-medical... (2020).
-
- Linden, A. & Fenn, J. Understanding Gartner’s Hype Cycles. http://www.ask-force.org/web/Discourse/Linden-HypeCycle-2003.pdf (2003).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

