Alternative oxidase encoded by sequence-optimized and chemically-modified RNA transfected into mammalian cells is catalytically active
- PMID: 33664504
- PMCID: PMC9750868
- DOI: 10.1038/s41434-021-00235-z
Alternative oxidase encoded by sequence-optimized and chemically-modified RNA transfected into mammalian cells is catalytically active
Erratum in
-
Correction: Alternative oxidase encoded by sequence-optimized and chemically-modified RNA transfected into mammalian cells is catalytically active.Gene Ther. 2024 Sep;31(9-10):528. doi: 10.1038/s41434-024-00473-x. Gene Ther. 2024. PMID: 39147867 Free PMC article. No abstract available.
Abstract
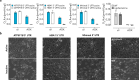
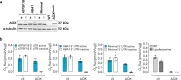
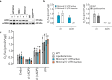
Plants and other organisms, but not insects or vertebrates, express the auxiliary respiratory enzyme alternative oxidase (AOX) that bypasses mitochondrial respiratory complexes III and/or IV when impaired. Persistent expression of AOX from Ciona intestinalis in mammalian models has previously been shown to be effective in alleviating some metabolic stresses produced by respiratory chain inhibition while exacerbating others. This implies that chronic AOX expression may modify or disrupt metabolic signaling processes necessary to orchestrate adaptive remodeling, suggesting that its potential therapeutic use may be confined to acute pathologies, where a single course of treatment would suffice. One possible route for administering AOX transiently is AOX-encoding nucleic acid constructs. Here we demonstrate that AOX-encoding chemically-modified RNA (cmRNA), sequence-optimized for expression in mammalian cells, was able to support AOX expression in immortalized mouse embryonic fibroblasts (iMEFs), human lung carcinoma cells (A549) and primary mouse pulmonary arterial smooth muscle cells (PASMCs). AOX protein was detectable as early as 3 h after transfection, had a half-life of ~4 days and was catalytically active, thus supporting respiration and protecting against respiratory inhibition. Our data demonstrate that AOX-encoding cmRNA optimized for use in mammalian cells represents a viable route to investigate and possibly treat mitochondrial respiratory disorders.
© 2021. The Author(s).
Conflict of interest statement
MS is a shareholder in a company set up to develop AOX-based therapies. CP and CR hold equity in Ethris GmbH. All other authors declare no conflict of interest.
Figures

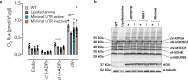
Comment in
-
Optimized expression of alternative oxidase.Gene Ther. 2022 Dec;29(12):653-654. doi: 10.1038/s41434-022-00340-7. Epub 2022 May 17. Gene Ther. 2022. PMID: 35577968 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

