Efficacy of rFVIIIFc versus Emicizumab for the Treatment of Patients with Hemophilia A without Inhibitors: Matching-Adjusted Indirect Comparison of A-LONG and HAVEN Trials
- PMID: 33664606
- PMCID: PMC7921628
- DOI: 10.2147/JBM.S288283
Efficacy of rFVIIIFc versus Emicizumab for the Treatment of Patients with Hemophilia A without Inhibitors: Matching-Adjusted Indirect Comparison of A-LONG and HAVEN Trials
Abstract
Purpose: Primary prophylaxis, using factor VIII replacement, is the recognized standard of care for severe hemophilia A. Recombinant factor VIII-Fc fusion protein (rFVIIIFc) and emicizumab, a humanized, bispecific antibody, are approved for routine prophylaxis of bleeding episodes in severe hemophilia A. These products have different mechanisms of action, methods of administration and treatment schedules. In the absence of head-to-head trials, indirect treatment comparisons can provide informative evidence on the relative efficacy of the two treatments. The aim of the study was to compare the approved dosing regimens for each product, rFVIIIFc individualized prophylaxis and emicizumab administered once every week (Q1W), every 2 weeks (Q2W) or every 4 weeks (Q4W), based on clinical trial evidence.
Patients and methods: The comparison was conducted using matching-adjusted indirect comparison since clinical evidence did not form a connected network. Individual patient data for rFVIIIFc (A-LONG) were compared with data for emicizumab (HAVEN trial program) for mean annualized bleeding rate (ABR) and proportion of patients with zero bleeds. Safety data reported across the analyzed treatment arms were tabularized but not formally compared.
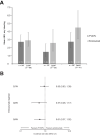
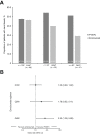
Results: After matching, no significant differences were observed between mean ABR for rFVIIIFc and emicizumab administered Q1W, Q2W or Q4W. The proportion of patients with zero bleeds was significantly higher with rFVIIIFc compared with emicizumab administered Q4W (51.2% versus 29.3%, respectively; odds ratio 2.53; 95% confidence interval 1.09-5.89); no significant differences noted when rFVIIIFc was compared with emicizumab administered Q1W or Q2W. The mean number of adverse events expressed per participant was 1.9 for individualized prophylaxis with rFVIIIFc and 3.7-4.0, 4.1 and 3.6 for emicizumab administered Q1W, Q2W or Q4W, respectively.
Conclusion: This indirect treatment comparison suggests that rFVIIIFc individualized prophylaxis is more efficacious than emicizumab Q4W, and at least as effective as more frequent emicizumab regimens, for the management of hemophilia A.
Keywords: annualized bleeding rate; antibodies; bispecific; comparative effectiveness research; efmoroctocog alfa; factor VIII deficiency; treatment outcome.
© 2021 Klamroth et al.
Conflict of interest statement
RK reports research funding and honoraria for consulting and lectures from Bayer, Biomarin, Biotest, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Takeda/Shire and Sobi. PW, SA and FD are employees of Creativ-Ceutical a consultancy company that received funding from Sobi for this research. ZH, JN, LAF, SL and ES are employees of Sobi. MDT reports speaking and consultancy fees from Amgen, HemaBiologics, Pfizer, Principia, Takeda, Grifols, Octapharma and Biomarin; consultancy fees from Novo Nordisk, Genetech and Roche; and trial investigator for Takeda and Spark Therapeutics. He has a private practice that offers in and out-patient consultation services and the CEO and CFO for Bleeding and Clotting Disorders Institute. The authors report no other conflicts of interest in this work.
Figures
References
-
- National Organization for Rare Disorders (NORD®). Haemophilia A. Available from: https://rarediseases.org/rare-diseases/hemophilia-a/. Accessed October 2020.
LinkOut - more resources
Full Text Sources
Other Literature Sources

